Publications Hub, EuroPerio, Treatment, Article
Bringing patients into the picture: Rethinking how we treat grade III furcations
28 July 2025
Grade III furcations are one of the most challenging defects that periodontists face in clinical practice. Priya Bahal, who was presented with the second prize in the Jaccard-EFP Research Prize at EuroPerio11 in May, describes the innovative research that she and her colleagues at King’s College, London, have carried out which gives equal weight to patient-reported outcomes and to the clinical evaluation of different techniques.
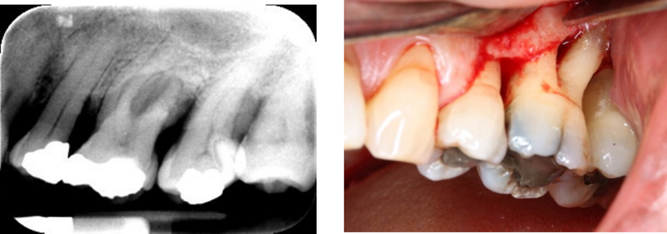
Treating advanced periodontal furcation defects, particularly grade III furcations, remains one of the most challenging clinical scenarios in periodontology. Despite being aware that the presence of a furcation involvement leads to a higher risk of tooth loss, there is still much variation in the literature regarding the most suitable approach or approaches to the most advanced grade III furcation defects.
Most published studies in this area are dated, focus on early-stage furcations, or use designs that do not reflect the stepwise, personalised care typically provided in clinical practice. Our feasibility trial set out to do something different: to transform the way clinical research is conducted, with a patient-centred focus throughout that attempted—as much as possible—to mirror the way clinical care is performed.
We combined rigorous clinical methodology with a forward-thinking design: a sequential, multiple assignment, randomised trial (SMART) design, which is new to the field of periodontal research. We sought to assess how molar teeth with grade III furcation involvement respond to surgical and non-surgical therapies, while being aware that this was the first time such a trial had been completed in periodontal research. Our study also broke new ground by incorporating patient-reported outcome measures (PROMs) into the definition of treatment success and we included a member of the public as a co-investigator throughout the research process.
Why grade III furcations?
Because of the complexity of grade III furcation-involved molars and the advanced bone loss that is present, many treatments are often rendered ineffective. These teeth are often deemed to have a poor long-term prognosis and are frequently extracted. This may be because of a lack of knowledge about potential treatment for such teeth, so we wanted to contribute to the literature to see if we could help provide information on how best to try to retain them, especially when strategic or patient-driven concerns make extraction less desirable. Our goal was not only to understand how these teeth respond to treatment but also to determine whether a personalized, adaptive trial design could be feasible in this clinical setting.
Why SMART?
Traditional randomised controlled trials (RCTs) assign a treatment, and the participants are then limited to that specific treatment arm. While this design has been imperative in providing evidence over the years, it should be noted that it does have several limitations. It uses a “one size fits all” approach, irrespective of individual variation, so it has limited external validity and is of limited benefit to those who are on the placebo or less-effective treatment arm. In today’s clinical practice, there is an overall move towards a more personalized approach as we shift towards more patient-centred care. Frequently, we start with minimally invasive therapies, we reassess and then escalate if needed. SMART designs are the first to mirror this logic.
In our study, 20 patients were firstly randomly allocated to either non-surgical periodontal treatment (NSPT) or open-flap debridement (OFD).
Participants were evaluated at a six-month time point and, based on their combined response (clinical and patient-reported) to treatment, they were deemed either a responder or a non-responder. The non-responders were then re-randomised to another, more involved treatment option.
The role of PROMs: more than a tick-box
A novel and particularly powerful aspect of our trial was the equal weighting given to patient-reported and clinical outcomes. To gain an understanding of the patient experience, participants answered three simple questions at each visit:
- Do they experience any pain from the tooth?
- Is there the presence of any bleeding?
- Is the tooth sensitive to heat or cold?
These responses, alongside conventional measures such as probing pocket depth (PPD), were used to determine whether a treatment could be considered successful. The table below shows how participants may have been deemed responders or non-responders at the six-month reassessment.
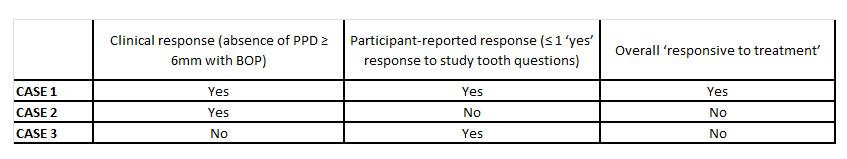
We found that there was agreement between clinical and patient-reported outcomes in 12 out of 20 patients (60%) at six months. This mismatch highlights how often clinicians may deem a treatment to be “successful”, while the patient remains dissatisfied, or vice versa. Including PROMs ensured that our outcome measure reflected not only what was recorded clinically but also—and crucially—what patients felt.
Patients as coinvestigators: insights from the inside
This novel trial was also the first in periodontology (to our knowledge) to include a “patient champion” as a coinvestigator. A member of the public with previous experience of dental disease helped shape the study design, reviewed patient-facing materials, and contributed to the article’s dissemination. His input offered valuable insights, especially in areas such as how the SMART design was explained to members of the public and how to most effectively gain an understanding of the patient experience.
This type of patient involvement aligns with emerging standards in clinical research but remains rare in dental trials. Yet it offers clear benefits: improved study relevance, better communication with participants, and increased external validity. It also challenges researchers to consider the outcomes that matter most to patients—not just those that look good in statistical tables.
What did we learn?
Clinically, our data showed that OFD led to greater reductions in periodontal probing depth (PPD) than NSPT: 2.3mm versus 1.4mm at six months, respectively. However, patient-reported success rates were similar across both groups. Uptake for a second surgical intervention was low, particularly among those who had already undergone surgery, highlighting the limits of re-randomisation within a trial and the idea of “participant fatigue”.
Most importantly, the SMART design proved feasible. While our recruitment rate was slower than anticipated, the retention was high, and patients were generally willing to be randomized. The use of a composite outcome combining PROMs with clinical metrics was informative but also introduced complexity, especially in cases where examiner judgement overruled strict protocol definitions. This suggests that future trials may benefit from weighting one domain rather than using the 1:1 ratio for clinical and patient-reported outcome measures or from considering the use of a simplified definition of success.
Where do we go from here?
This was a feasibility study, not a definitive trial, and its limitations include the small sample size and the single-centre setting, and it is also important to acknowledge the protocol deviations. But equally as important, we would like others to see that our findings really do open new doors. We now have the first clinical comparative data for surgical versus non-surgical treatment of grade III furcations within a SMART framework, and a demonstration that personalized-medicine approaches are possible in the world of dental and periodontal research.
We hope this study encourages researchers to adopt more patient-centred designs and to consider PROMs not as secondary outcomes but as coequal partners in defining treatment success. We feel that including patients in the research process and allowing them to have a voice should be a key aspect of future studies in periodontology and beyond.
Select bibliography
Almirall D, et al. (2014). Introduction to SMART designs for adaptive interventions.Transl Behav Med.
Alshamsi M, et al. (2021). Primary outcomes in periodontal RCTs: A systematic review. J Clin Periodontol.
Dommisch H, et al. (2020). Resective surgery for furcation involvement—Systematic review. J Clin Periodontol.
Loos BG & Needleman I (2020). Endpoints of active periodontal therapy.J Clin Periodontol.
Needleman I, et al. (2023). Outcomes of periodontal therapy: A co-created review.Periodontology 2000.
Nibali L, Zavattini A, et al. (2016). Tooth loss in molars with and without furcation involvement—A systematic review.J Clin Periodontol.
Sanz-Sánchez I, et al. (2020). Access flap vs subgingival debridement.J Clin Periodontol.
Tonetti MS, et al. (2018). Staging and grading of periodontitis: New classification. J Periodontol.
Biography

Priya Bahal is a specialist periodontist who completed her periodontal training at Guy’s Hospital and King’s College London. She gained her Bachelor of Dental Surgery degree from the University of Bristol in 2010 where she later completed her Master’s degree with research on dentine hypersensitivity.
She has numerous publications and has presented at international level, most recently for the Jaccard-EFP Research Prize at EuroPerio 11. She is based in Harley Street, London and in Essex, UK.
See also:
JCP Digest: How do clinical and patient-reported outcomes compare in treatment of teeth with grade III C furcation?
Journal of Clinical Periodontology: 'Clinical and Patient-Reported Outcomes in Grade III Furcations: A Randomized Feasibility Trial With SMART Design', Journal of Clinical Periodontology 52(7), July 2025. https://doi.org/10.1111/jcpe.14134
News report: 'Jaccard-EFP Prize awarded at EuroPerio11'




