
Issue No. 146
Summarized from Journal of Clinical Periodontology, Volume 53, Issue 2, February 2026, 268–285
Editor: James Deschner, chair, EFP scientific affairs committee
Does collagen membrane improve reconstructive surgery with xenogeneic bone in treatment of peri-implantitis?
Authors: Carlotta Dionigi, Erik Regidor, Anna Trullenque-Eriksson, Jan Derks, Mariano Sanz, Alberto Ortiz-Vigón
Background
Peri-implantitis therapy can lead to favourable short-term improvements. However, medium- and long-term studies have reported substantial rates of disease recurrence and a frequent need for additional treatment. Regular supportive peri-implant care and good oral hygiene following surgery are associated with reduced recurrence and improved long-term outcomes. Other parameters, such as smoking and implant-surface characteristics, have also been identified as predictors of treatment outcomes.
When clinicians surgically manage intrabony peri-implant defects, different approaches can be selected, including open-flap debridement, resective therapy, and reconstructive surgery. So far, controlled studies have not consistently shown a clear short-term superiority of one approach over another.
In reconstructive procedures, bone substitutes are often combined with barrier membranes to support bone regeneration, yet evidence for the additional clinical benefit of membranes is limited. A previous one-year report from the trial summarized here said that the use of adjunctive membrane did not result in significantly different clinical or radiographic outcomes compared with grafting alone. It is possible that differences may become apparent only over longer observation periods. Therefore, mid-term follow-up data are needed to clarify whether membranes provide any meaningful advantage when used in combination with bone-substitute materials in reconstructive peri-implantitis surgery.
Aim
To evaluate whether the adjunctive use of a resorbable collagen membrane with a xenogeneic bone substitute during reconstructive surgical treatment of peri-implantitis with intrabony defects, improves three-year outcomes compared with xenogeneic grafting alone.
Materials & methods
Study design and participants:
- Randomized controlled clinical trial with two parallel arms, conducted at a single clinic.
- Forty-three patients were enrolled according to inclusion criteria (e.g. at least 18 years old, not smoking more than 10 cigarettes per day). One implant per patient diagnosed with peri-implantitis (PPD ≥7 mm, BOP/SOP, marginal bone loss ≥3mm) was included in the analysis.
- Defect eligibility: Peri-implant intrabony defect depth ≥3mm and width ≤4mm (radiographically assessed and confirmed intra-surgically) with at least two walls (mesial and distal; no requirement for a buccal/lingual wall).
Intervention protocol:
- All participants received oral-hygiene instructions, supra/sub-marginal instrumentation, and prosthetic adjustments as needed.
- Antibiotic prophylaxis: Amoxicillin 750mg twice daily for 10 days starting three days before surgery.
- Surgical procedure: Granulation tissue excised with a titanium curette and implant surface decontaminated using a rotating titanium brush.
- Patients were randomly assigned to one of two interventions:
- Control: Xenogeneic bone substitute (Bio-Oss collagen).
- Test: Same graft plus a resorbable collagen membrane (Bio-Gide) secured with pins or sutures (or both).
Follow-up and assessments:
- Supportive peri-implant care every six months for three years (hygiene reinforcement and instrumentation).
- Blinded evaluations at baseline, six, 12, and 36 months. At each visit, probing pocket depth (PPD), bleeding on probing (BOP), suppuration on probing (SOP), and plaque were recorded at four sites per implant. Mid-buccal marginal mucosal level (REC) and keratinised-mucosa width (KM) were also measured.
- Radiographic marginal bone levels were measured at baseline, 12, and 36 months.
- Patient-reported outcomes assessed at baseline, two weeks, 12 months, and 36 months.
- Primary outcome: composite measure consisting of PPD≤5mm, absence of BOP/SOP, and REC change ≤1mm.
- Secondary outcome: Disease resolution defined as PPD≤5mm, BOP≤1 sites, and complete absence of SOP as well as radiographic and patient-reported outcomes.
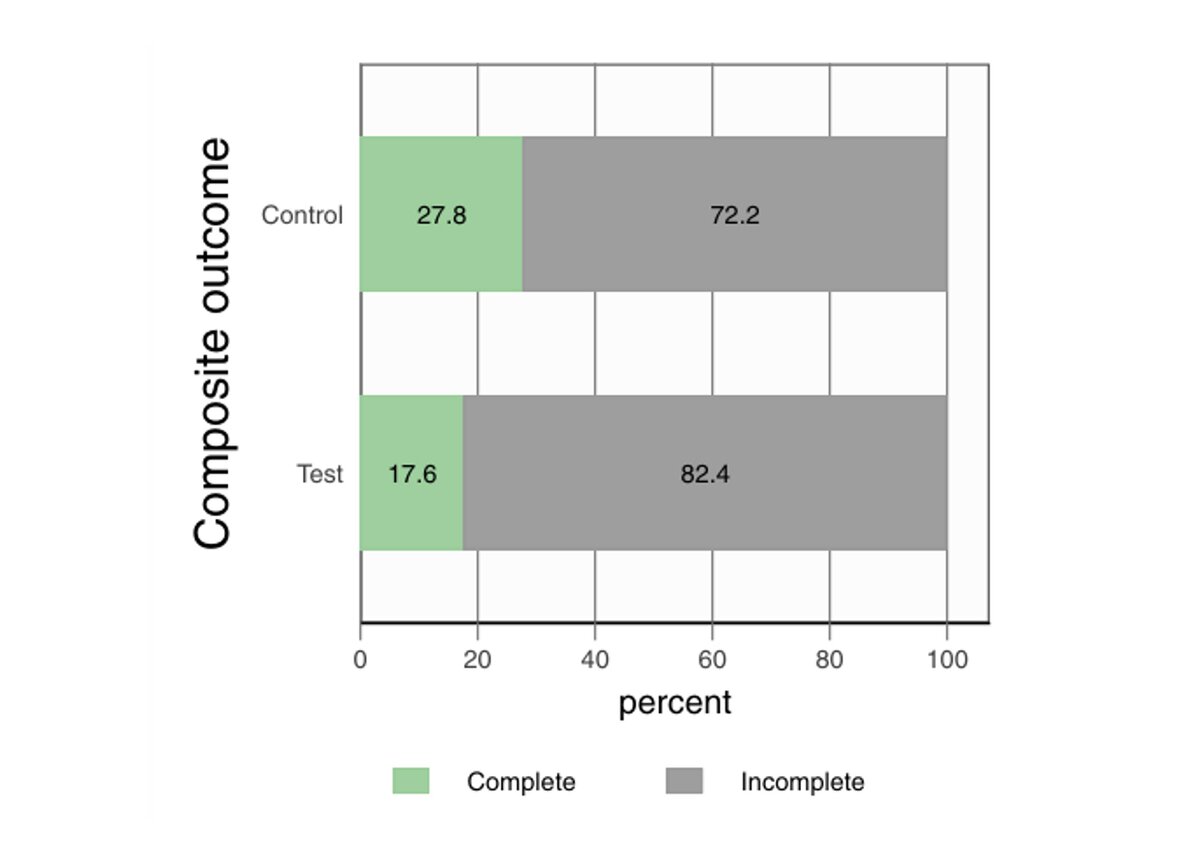
Results
- Of the 43 enrolled participants, eight were lost to follow-up, two experienced implant loss (one per group), and one patient (test group) underwent surgical retreatment. A total of 32 patients (17 control, 15 test) participated in the 36-month evaluation.
- The composite endpoint was achieved in only 22.9% of implants overall, with no significant difference between groups (control: 27.8%; test: 17.6%).
- Disease resolution was achieved in 28.6% of implants overall (control: 27.8%; test: 29.4%), again showing no significant inter-group differences.
- At the implant level, 53.1% exhibited a maximum PPD ≤5mm, 34.4% showed no BOP, 78.1% showed no SOP, and 53.1% had a buccal REC ≤1mm at 36 months.
- Overall clinical improvements from baseline to 36 months included mean PPD reduction of 3.6mm, BOP reduction of 37.5%, buccal REC increase of 0.5mm, and KM decrease of 1.1mm. No statistically significant differences were observed between groups for any parameter.
- Radiographic bone gain (MBL improvement) was achieved to varying degrees in 71.8% of cases, with an overall mean MBL change of 1.0mm (bone gain). Again, with no significant difference between groups.
- Between 12 and 36 months, there was a decline in clinical stability, reflected by higher BOP and SOP prevalence, a moderate increase in PPD, and a lower proportion of implants meeting composite success criteria.
- Patient-reported outcomes at 36 months showed high satisfaction levels regarding therapy, with no significant intergroup differences.
- Although not statistically significant, fully compliant patients tended to show more favourable outcomes than partially compliant patients (32% vs. 13%).
Limitations
- The small sample size (n=43 at baseline; n=32 at three years) resulted in limited statistical power (≈49% for marginal bone-level change and ≈11% for the composite outcome), increasing the risk that a small-to-moderate membrane benefit may have been missed.
- A standardized six-month supportive care interval may have been insufficient for some patients, as reflected by increased plaque scores over time.
- Despite randomization, defect characteristics were unbalanced, with more non-contained defects in the test group (81% vs. 54.5%), potentially confounding comparisons. Sensitivity analyses including only non-contained defects suggested more favourable responses at test sites.
- The a priori sample-size calculation was based on marginal bone-level change rather than the stricter composite clinical endpoint.
- The three-year results demonstrate that, while reconstructive surgery leads to significant improvements in probing depths and bone levels, complete success remains a clinical challenge.
- Within the limitations of the study, the adjunctive use of a resorbable membrane in combination with a xenogeneic bone substitute did not result in additional clinical or radiographic benefits in the reconstructive treatment of peri-implantitis.
- Although reconstructive surgery proved safe and effective in reducing disease severity, complete disease resolution remained difficult to achieve, with only about 25% of treated implants fulfilling the strict composite success criteria. These findings suggest that mid-term success may depend more on defect morphology and strict adherence to supportive therapy than on the use of a barrier membrane.
The addition of a resorbable membrane to a bone substitute is unlikely to influence mid-term outcomes of reconstructive peri-implantitis surgery. Clinical emphasis should therefore be placed on appropriate case selection, defect morphology, and consistent supportive peri-implant care, which appear to be more critical for achieving and maintaining clinical stability.
Rapporteurs: Bertha Demetriou, Daniela Matanes, Tarek Matanes, Yasmin Saba, Ido Georgy, Lir Abergil, and Yarden Berg, supervised by Ofir Ginesin
Affiliation: Postgraduate programme in periodontology, Health Care Campus Rambam, Israel
With kind permission from Wiley Online Library. Copyright © 1999-2026 John Wiley & Sons, Inc. All rights reserved




