Treatment, Article, Classification & Guidelines
What is the evidence base for peri-implant treatments?
31 August 2025
Peri-implantitis has emerged as a formidable threat to public oral health for two main reasons: it can manifest clinically within only a few years of implant function, it affects more than 10% of people with dental implants, and it is often resistant to treatment, resulting in clinical implant failure. As a result, patients often require expensive surgical intervention and bone reconstructive procedures—but only around half of these peri-implantitis treatments are successful.
In a special three-part article, Mia Rakic, elected member of the EFP executive committee and a member of the federation’s scientific affairs committee, introduces the problem of peri-implantitis. Focusing on a specific case, Georgios Kotsakis, Paul Fletcher, Paul Rosen, and Dennis Tarnow offer their professional insights into which peri-implantitis treatments are most effective and reliable. Finally, Mia Rakic discusses the same case in the light of the EFP’s recent S3-level clinical practice guideline for the treatment of peri-implantitis.
I. Peri-implantitis as a problem
By Mia Rakic
Peri-implantitis is an emerging public-health problem because of its increasing prevalence and lack of predictive treatment, and its association with major negative health and socio-economic impacts. For years, peri-implantitis was considered as a pathological counterpart of periodontitis at implants, which is why preventive, diagnostic, treatment, and maintenance strategies were simply adopted form periodontology.
But we are now witnessing the fact that periodontal clinical protocols show limited effectiveness when applied to dental implants, and the two major reasons for that are lack of periodontal ligament and implant surface-related factors. Lack of periodontal ligament entails to increased vulnerability of the implant, a fast-progressing and asymptomatic course of disease, and a decreased regenerative potential of peri-implant tissues.
Meanwhile, the implant surface is challenging in terms of decontamination and may undergo physicochemical alterations when exposed to infection and instrumentation with inappropriate instruments and technique (Kotsakis & Gansean, 2025). For example, it has been shown that under inflammatory conditions, the abrasion caused by titanium brushes can lead to loss of titanium passivation and increased titanium particle release (Kotsakis et al., 2024; Rakic et al., 2022), which can cause intense inflammatory activity in the peri-implant tissues.
The XVIII edition of the European Workshop in Periodontology in 2022, organized by the European Federation of Periodontology, was devoted to the development of an S3-level clinical practice guideline for the prevention and treatment of peri-implant diseases (Herrera et al., 2023). The EFP guideline was based on high-quality scientific evidence and drawn up according to a strict methodology, to serve as a global guide for clinical strategies for peri-implantitis.
Nonetheless, clinicians face a formidable challenge with peri-implantitis because they are dealing with a pathology that lacks a standard treatment, while the validation and clinical implementation of new protocols takes time. In this context, different treatment plans proposed by experts based on their clinical experience and emerging scientific evidence are valuable for clinicians.
The emerging scientific evidence is particularly concerned with factors related to the implant surface and its contamination, and iatrogenic-induced alterations might be implicated in destruction patterns, treatment success, and the recurrence of peri-implantitis (Wachi et al., 2015; Kister et al., 2017; Eger et al., 2017). In this sense, different clinical opinions allow us to better indicate the lack of standard protocols and the advantages of innovative strategies that might be of help in adapting some everyday practices for better outcomes even before the implementation of new technologies and therapies. In this featured article four international experts in peri-implantitis treatment have been invited to share their current practices for peri-implantitis treatment.
II. Optimising treatment strategies
By Georgios Kotsakis, Paul Fletcher, Paul Rosen, and Dennis Tarnow
The need for optimal treatment strategies has led to a vast array of peri-implantitis implant regimes that are often either empirical or driven by knowledge of effective dental-treatment strategies. But implant surfaces are nothing like those of teeth, and it has emerged that dental antimicrobial treatments are largely ineffective and potentially hazardous when applied around implants (Esposito et al., 2012; Kotsakis & Olmedo, 2021).
As implant research advances, new implant-specific translational in vitro and pre-clinical research models are being established that have provided critical data about the key components of peri-implant regeneration. Across published studies there is overall agreement that there are three key pillars of peri-implant regeneration:
- Implant surface decontamination for biofilm reduction. It is crucial to reduce the microbial biofilm biomass from the peri-implant sulcus. But elimination is not necessary – indeed, elimination strategies with abrasive mechanical instruments, such as titanium curettes and ultrasonic instruments, have been shown to be not only ineffective, but also hazardous. It is now well established that when the implant surface is abraded, the titanium passivation layer responsible for osseointegration and the prevention of implant corrosion is possibly irreparably lost. Thus, the reduction of the biofilm needs to reach a certain critical load beyond which the immune surveillance in the peri-implant tissues can take over and re-establish a homeostatic equilibrium by eliminating pathogens and keeping peri-implant commensal bacteria under surveillance.
- Maintenance of implant surface integrity. It has now been established that when the titanium passivation layer is abraded by instrumentation and the implant surface is exposed to anaerobic inflammatory conditions—such as those that exist in the peri-implant pocket—the titanium may not return to passivation. Instead, this can be the beginning of a significantly accelerated breakdown of titanium and the dissolution of titanium particles in the microenvironment.
- Improvement of the osteoregenerative potential. Peri-implant defects present a regenerative challenge as, unlike periodontal defects, the cellular reparative mechanisms face previously contaminated biomaterial surfaces that are not as supportive of tissue integration.Several regenerative biomaterials that can be either osteoinductive or osteostimulative have been used and in vitro data suggest that they can at least partially restore titanium cytocompatibillty. The challenge, therefore, lies in the selection of the most cost-effective strategy among all candidate biomaterials.
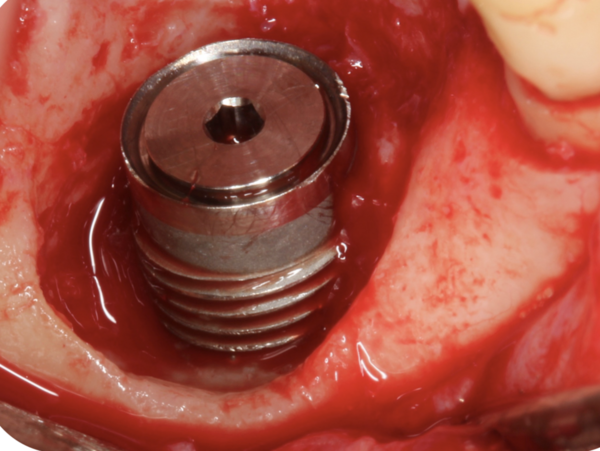
A clinical case
The four authors of this article – well-established peri-implantitis clinical researchers – offer their insights on how to address these three pillars of peri-implant regeneration by focusing on a specific clinical case:
- 52-year-old male, non-smoker.
- Maximum pocket depth (PD) of 7mm disto-buccal.
- Bleeding on probing (BoP) circumferentially around the implant without suppuration. Probing depths on #45, #46 are <4mm.
- Diagnosis of peri-implantitis #47 according to the 2018 Classification of Peri-implant Diseases and Conditions.
- Severity classification: Moderate, Type A peri-implantitis according to Rosen et al. 2022.
- Defect classification: Class Ie according to Schwarz et al. 2007
The patient presented with the clinical signs and symptoms described. The cement-retained crown was retrieved, and, after removal of granulation tissue, the corresponding clinical image was obtained which revealed the extent and configuration of the defect.
Forum: How to treat this case
1. Implant surface decontamination for biofilm reduction
How would you perform it in this case and why? Any considerations in the decision to use one technique rather than another?
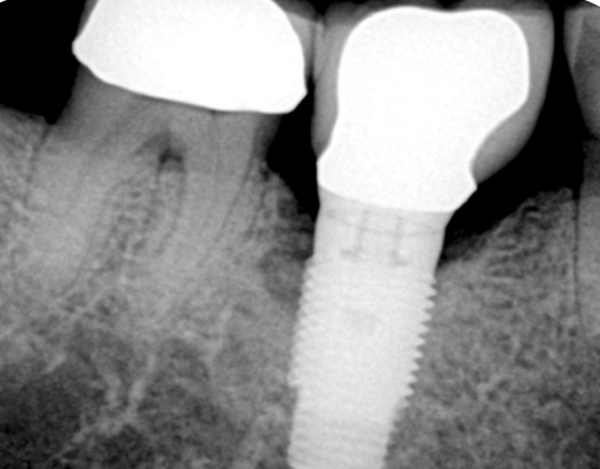
Georgios Kotsakis: Recent research seems to point to this first pillar as being the most critical one. If the implant surface becomes mechanically abraded and loses its passivation, then it stops being biocompatible and this hinders tissue integration. Therefore, in an implant surface that is not capable of osseointegration, even the most osteoinductive biomaterial would not yield positive outcomes. In fact, there is data suggesting that a single occurrence of mechanical abrasion of the submucosal implant surface can lead to a massive release of titanium into the peri-implant plaque at a very high rate over the following eight weeks (Daubert et al. 2023). Based on preclinical evidence I would avoid any chemotherapeutic agent with non-selective cytotoxicity – such as Chlorhexidine – as this can adsorb on the titanium surface and significantly reduce the chances of re-osseointegration (Kotsakis et al., 2016, Qian et al., 2024). My preference would be to use the "shoe-shine" technique with a gauze soaked in saline after the removal of granulation tissue followed by irrigation with a pressurized water jet, which seems to be the most effective and non-abrasive approach to biofilm removal.
Paul Fletcher: Whether bacteria, cement, genetics, occlusion, smoking, titanium corrosion, or an inflammatory host response to a foreign implant body are sole or coinciding factors that contribute to the development and progression of the peri-implantitis lesion, treatment would be directed towards detoxifying the implant surface and the surrounding bone and soft tissue. A plastic curette – categorized as a mechanical, minimally abrasive contact instrument – would be used to disrupt or remove the biofilm colonies and the loose titanium particles and ions associated with the surface of the implant, bone, and soft tissue. A glycine powder air polisher –considered to be a mechanical, non-abrasive, non-contact instrument – would then be utilized to further decontaminate the implant and the surrounding bone and soft tissue. The implant surface would then be treated chemically by burnishing it with a 0.25% sodium hypochlorite (NaClO) solution and then a 1.5% hydrogen peroxide (H2O2) solution to kill bacteria, followed by burnishing and thorough rinsing with sterile saline to further dilute or remove any residual endotoxin, titanium particles, or other contaminants. A final empirical step would be to repeatedly fill a Monojet syringe with air and use the tip to go around and express air into the acute angles of the implant threads and microtopography of the implant surface and thereby possibly contributing to the repassivation of a titanium oxide layer.
Paul Rosen: The surface decontamination protocol for this case must consider not only what might be effective in eliminating any biofilm present, but also the approach after all biofilm is eliminated. The defect classifications of both Schwarz and colleagues (2007) and Rosen and colleagues (2022) place the implant in the centre of the alveolar ridge with a circumferential defect. The potential exists for a regenerative approach with a high degree of confidence for success. Since it has been possible to remove the crown and place a cover screw, the implant can be submerged for healing, which will enhance the ability to provide a successful regenerative solution (Schwarz et al., 2006). That said, the buccal bone is at a more apical level than that on the lingual, and the alveolar ridge is narrower in its coronal aspect than apically. It is my belief that with any regenerative approach, complete coverage of the implant body up to the platform will be difficult to achieve. If complete bone coverage is reached, it will not be with a bone width of 1.5 -2 mm. which has been shown to be critical for maintaining the result (Monje et al., 2019; Grunder et al., (2005). This will be further challenged by an inability to have platform switching for the restoration as depicted in the radiograph with the crown in place. This would also suggest that the most coronally regenerated hard tissues near the platform will neither be possible nor sustainable, as it will be difficult for them to resist any inflammation. If the bone is not maintained up to the platform, the patient will be faced with at least the re-exposure of the coronal aspect of this dental implant’s rough surface and perhaps even one or two of the threads on the buccal. My approach would include two aspects: (1) mechanical: a mechanical contact abrasive approach using implantoplasty, (2) chemical: using a non-selective cytotoxic agent in citric acid (pH=1 50% saturated solution).
Dennis Tarnow: I now use a combination of contact and chemical decontamination. After debridement of the granulomatous tissue (which has already been performed in the photo supplied), I would first use peroxide to chemically detoxify the lesion, followed by two different mechanical methods of debridement. First, I would use a series of carbide burs to perform implantoplasty both supra- and subcrestally. This ensures the removal of the remaining rough surface that is filled with bacteria and biofilm. Note that this approach differs from most of the literature, which says that only the supracrestal part of the implant should be smoothed. The first article on doing this type of subcrestal implantoplasty has recently been published, with case reports. This certainly needs to be further validated by controlled long-term studies. I then use Ti-Brushes to further ensure that the surface is clean.
2. Maintenance of implant-surface integrity
How would you factor in the preservation of implant-surface cytocompatibility and preservation of the titanium passivation layer to prevent future release of titanium particles?
GK: Preservation of implant surface cytocompatibility is critical for osseointegration. At present, there are no established ways of enhancing surface cytocompatibility, thus the goal should be to maintain it. By avoiding abrasive means of cleaning, such as the titanium curette, the passivation layer is maintained as best as possible.
PF: As osseointegration has been shown to occur against a clean, passivated titanium surface, all decontamination therapy is directed towards reestablishing and maintaining this environment. The plastic curette used for implant debridement has been shown to be minimally abrasive (Schmage et al., 2012), unlike titanium and stainless-steel curettes, titanium brushes, and all ultrasonic tips, which have been shown to cause a greater number of titanium particles to be released from the implant (Delgado-Ruiz & Romanos, 2018).NaOCl is a bactericidal oxidant with a basic pH that will not corrode titanium at low concentrations (Slots, 2012; Kotsakis et al., 2016), as opposed to using more acidic chemicals that can corrode a titanium implant surface. H2O2 duplicates the effects of NaOCl, has been shown effective in the removal of lipopolysaccharide endotoxin, and can contribute to the reformation of the oxide layer (Walivaara, Lundstrom & Tengvall, 1993). Implantoplasty –more apt to be utilized when the implant threads are outside the buccal and lingual plates of bone – would not be appropriate for the implant in this confined defect to avoid the possible spread and retention of residual titanium particles in the soft tissues and bone of the infrabony defect. Lasers, used at the appropriate settings, have been shown to successfully clean an implant surface and could also be used in the decontamination process. During at-home maintenance, the patient should be directed to brush the implant twice per day using a fluoride-free dentifrice to avoid the possible negative long-term corrosive impact of the repeatedapplication of an acidic pH fluoride toothpaste (Schiff et al., 2022). Additionally, they should be requested to use an oral irrigator, containing half a teaspoon of bleach in a 500ml reservoir, three times per week around all implants and under all frameworks to try to disrupt the biofilm colonies and keep them at a sub-inflammatory concentration.
PR: My answer is partially predicated on both my experience and by what is available in my armamentarium. I have personally witnessed in my own practice that using implantoplasty followed by citric acid for surface decontamination prior to osseous grafting has been able to successfully decontaminate the implant’s affected surface, as has been substantiated in a study conducted by Dennis Tarnow and me (Rosen & Tarnow, 2023). In adopting implantoplasty as my contact abrasive in this approach, I would use carbide finishing burs that are 12 fluted (Brasseler USA, Savannah, Georgia, USA), which will remove the outer surface of the dental implant leaving a surface that may approach a machined texture and not one that is “polished.” El Chaar and colleagues (2020) used SEM to look at various chemotherapeutic and mechanical methods for surface decontamination of infected dental implants retrieved from humans – but only implantoplasty demonstrated complete biologic debris removal. Lin and colleagues (2022) performed a systematic review and meta-analysis that investigated the impact of implantoplasty with or without regenerative procedures on treatment outcomes for peri-implantitis. The data demonstrated that implantoplasty’s use – whether with a regenerative or non-regenerative approach – resulted in a high implant survival rate and that the former showed greater bone fill. The endpoint of implantoplasty in this case is to achieve a machined-like surface and not one that is polished. This distinction is important as polished surfaces are not conducive to achieving osseointegration (Hermann et al., 2011). Beyond the benefit of implantoplasty creating a surface free of contamination, which may allow re-osseointegration to occur, it will also provide better cleanability as not all regenerative efforts meet with 100% coverage of the dental implant. The reason that citric acid follows the implantoplasty relates to the work of Htet and colleagues (2016), who demonstrated – through histomorphometry in a beagle-dog study – that the combined use of a bur followed by citric acid achieved endpoints of bone-to-implant contact that exceeded the use of a bur alone and were also better than photodynamic therapy and Er:YAG laser for surface decontamination. Additionally, several case series (Froum et al., 2012; Froum & Rosen, 2014; Froum et al., 2015) demonstrate the use of citric acid as part of a treatment algorithm for surface decontamination on dental implants with successful regenerative outcomes, while an in vitro study (Rosen et al., 2018) has substantiated the positive outcome of citric acid’s use in a decontamination protocol for surfaces infected by peri-implantitis; as human osteoprogenitor cells were able to attach and proliferate after decontamination, an endpoint may be reached where osseoreintegration might be possible. Concern has been raised that citric acid may cause corrosion and hinder implant passivity, but research has shown this concern to be misplaced (Froum & Rosen 2014; Verdeguer et al., 2022).
DT: This surface can be removed using implantoplasty. It is still questionable as to whether the oxide does or does not reform after this new surface is exposed to air and oxygen. Passivation and the release of titanium ions may not be critical, since osseointergration can occur with other metals besides titanium, even including implants made of gold (Abrahammson & Cardaropoli, 2007). It is more important that the remaining bacteria and their dead cell walls (endotoxin) are properly and thoroughly removed, otherwise no healing will be effective.
3. Enhancing osteoregenerative potential
What regenerative biomaterial choices and/or biologics would you make for this case and why?
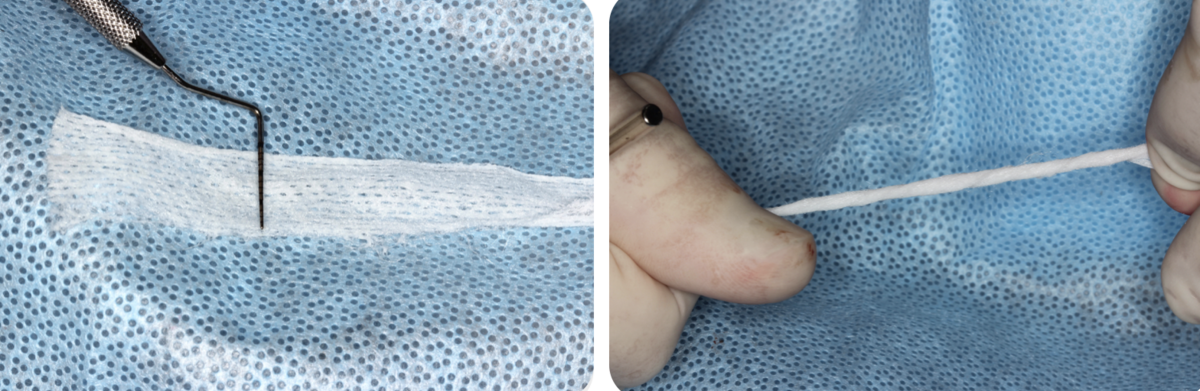
GK: Because of the configuration of peri-implant defects – that most often present as "saucer-like” defects with a large area of bone volume loss circumferentially of the implant – the regenerative potential is lower. The distance that nutrients have to cover via the circulation to jump to reach the implant surface in the centre of the defect is often beyond the regenerative potential of an osteoconductive scaffold. Not only that, but the implant surface itself is not as cytocompatible as it was at the time of placement after cleaning (Kotsakis et al., 2016). There are data suggesting that osteostimulative grafts –specifically calcium phosphosilicate – can partially restore titanium surface cytocompatibility by actively recruiting more osteoblasts to it during early healing phases (Karoussis et al., 2018). Thus, my first choice would be to use calcium phosphosilicate morsels (Novabone LLC, Alachua, FL) combined with locally harvested autogenous bone chips. Alternatively, there is clinical information that amelogenin in enamel matrix derivative can enhance peri-implantitis treatment resolution (Isehed et al. 2018), so in many cases I use a combination of allograft with enamel matrix derivative to treat these defects. This combination has the advantage of forming a viscous "sticky" compound that is easily adapted into the defect and provides good mechanical stability. Unless there is significant damage of the buccal or lingual plate that compromises mechanical stability during early healing, a membrane does not seem to provide significant advantages. Therefore, I do not routinely employ one in circumferential defects.
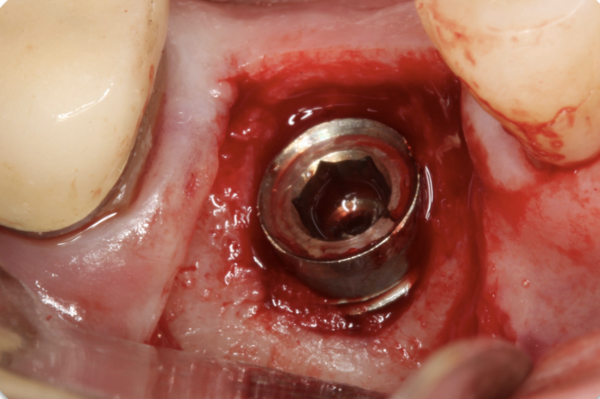
PF: Clot stabilization and its subsequent organization is imperative to achieve osseous regeneration (Gomes et al., 2019). In the relatively narrow circumferential infrabony defect in this case – where the entire implant is within the confines of the bony walls of the ridge – clot stabilization can be achieved by utilizing defect degranulation, implant surface detoxification, and implant submergence with a replaced flap. To maximize the possibility of obtaining an optimal result, a bone-graft combination (50:50 mix of mineralized cortical/cancellous bone allograft and bovine bone xenograft) (Abedi et al., 2020) would be placed in the defect to help stabilise the clot and to act as a scaffold for the conduction of osseous elements into the defect, as the different graft materials sequentially resorb over time (cancellous bone, followed by cortical bone and eventually by the xenograft). A resorbable collagen membrane would be placed over the grafted defect as a barrier and the gingival flap would be replaced to cover and protect the site during healing. Biologics – such as enamel matrix derivative and platelet-derived growth factor – have been shown to stimulate bone and soft-tissue growth (Miron et al., 2016) and could also be utilized in this case. However, in this confined and treatable defect, it does not seem necessary to alter what has been shown to be a relatively successful and predictable treatment protocol (Salle et al., 2023).
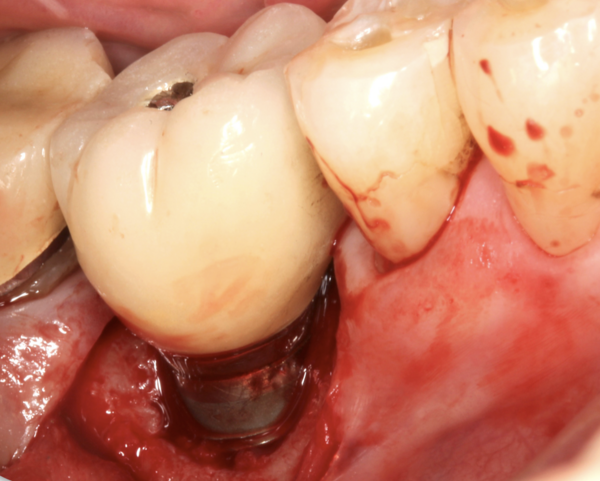
PR: The selections for regeneration would include the use of a biologic and several biomaterials. The biologic I have employed following treatment with citric acid and rinsing it off the implant surface is recombinant human platelet derived growth factor-BB (rhPDGF-BB). This is being used off-label, as I would strictly apply it to the implant surface without adding it to the carrier graft with which it is packaged. The reason for this is twofold. First, rhPDGF-BB is a hydrophilic agent, and we are trying to encourage having a hydrophilic surface on the implant that will be clot-stabilising. Second, this particular biologic might help in the recruitment of blood supply as it up-regulates VEGF (Cooke et al., 2006). Next, I would use a bone-replacement graft that is a combination of demineralized freeze-dried bone allograft fibres along with a particulate freeze-dried bone allograft. In terms of compatibility of graft products with human cells, the allografts have been far superior when it comes to attachment and proliferation of cells versus many of the other commercially available materials (Lallier et al., 2001). While historically, the graft materials have been formulated as particulates, more recently the tissue-banking industry has focused on processing those that get demineralized as fibres. This enables the grafts to be more uniformly demineralized throughout to enhance their inductivity to the point where they rival grafts that are combined with mesenchymal cells (Abedi et al., 2020). The fibre form of the demineralized allograft and the freeze-dried bone particulate are better contained at the site and provide very good space maintenance and scaffolding for the clot. The specific tissue phenotype will determine which type of barrier should be selected. In this case, my limited view is that the soft tissues on the buccal aspect would be adequate and so there appears to be no need to augment them with either a soft tissue autograft or allograft. In this instance, I would use a barrier that comes from amnion-chorion material to further contain the graft material and quite possibly have some intrinsic growth factors to further aid the regenerative outcome (Imamura et al., 2022). As these barriers are so thin, they minimize the space that they might take up, thereby allowing for more graft material to be placed.
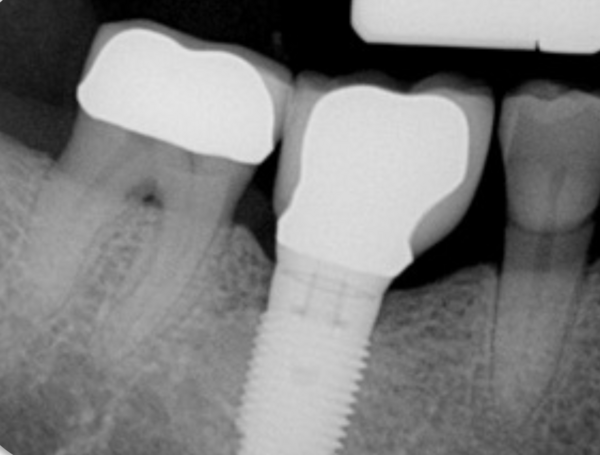
DT: Placing a bone graft with the use of a membrane appears to help the level of radiographic bone gain in the infrabony defect compared with simply leaving the defect debrided alone (with implantoplasty performed in both cases). It should be noted that the implantoplasty that has been performed and reviewed (Cho-Ying Lin et al., 2022) was only supracrestal smoothing and not subcrestal, as I am now recommending in this type of case. Also, the use of biologics may enhance the early repair and healing, but this has not been shown to modify the long-term effects beyond what is seen by adding a graft with a membrane.
Discussion and conclusions
While the dental-implant community is gaining more insight into the multiple factors that contribute to the initiation and progression of peri-implant disease, its detailed pathogenesis is still not fully understood. The initial inclination was to simply attribute the bacteriologic mechanism associated with the development of periodontitis to implants, but it now seems to be significantly more complicated than that. A dental implant is undeniably biocompatible but it also not native to the tissues – it is an osseointegratable biomaterial that does not elicit a foreign-body response. The implant is thought to exist in a state of equilibrium, until certain factors emerge that upset that equilibrium.Nonetheless, when an implant is mechanically damaged or otherwise corroded, it demonstrates pro-inflammatory behaviours in the tissues, mainly through its breakdown in the form of titanium particles.
Titanium, while long known to be a biocompatible metal, has also been shown to corrode in the oral environment. While its particles and ions can elicit a concentration-dependent inflammatory response, this inflammation does not manifest clinically in all patients. Recent data from in vitro and human studies suggest that titanium corrosion may be mostly iatrogenic through the abrasive treatment of implant surfaces.. Notably, although abrasive mechanical implant surface treatments such as implantoplasty and ultrasonic cleaning can remove biological debris from the implant surface in vitro (El Chaar et al., 2020), the abrasive cleaning causes irreparable damage to the implant passivation layer under inflammatory conditions (Kotsakis et al., 2024). The abrasive mechanical damage and loss of titanium passivation lead to the continuous release of implant-derived titanium microparticles that accrue in the peri-implant tissues and demonstrate proinflammatory and osteoclastic activities, which lead to significant bone loss in vivo (Eger et al. 2018). Thus, newer approaches focus on non-abrasive cleaning of the intrabony implant component that may in fact lead to re-osseointegration in humans (Fletcher et al. 2017).
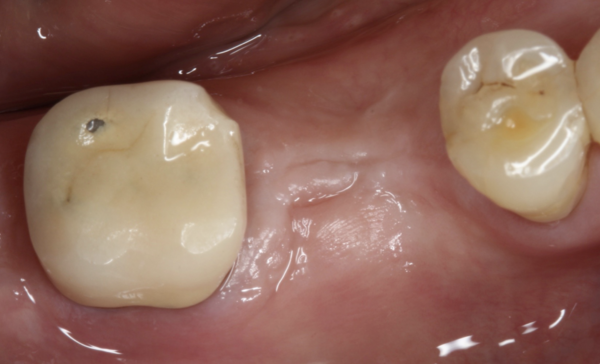
There are numerous interventions for the treatment of peri-implantitis, but there is still no reliable evidence as to which is most effective.However, there are several principles that practitioners should strongly consider when treating a peri-implantitis lesion. In the case presented here (whose resolution is shown in Figure 3 along with the treatment protocol), the only intervention to clean this implant surface consisted of cotton pellets with saline. This is counter-intuitive compared with the calculus-removal paradigm in periodontal treatment. However, calculus is very rarely found on micro-rough acid-etched implant surfaces. As a result, the intent is not to abrade mineralized deposits but rather to wash away the sticky biofilm away. Thus, simpler approaches may not only be more effective than abrasive mechanical treatments but can also reduce the release of titanium particles.
These particles in the long-term may contribute to the inflammatory burden that compromises healthy implant homeostasis. Clinicians need to be reminded that implants are not passively bioinert but are in a dynamic state of osteoimmune equilibrium, which can be compromised by titanium microparticles and plaque biofilms (Albrektsson et al., 2023).
This is not to say that if the other pillars of successful treatment are maintained there can be no resolution. In fact, there are multiple case reports and case series of successful regenerative treatment even in cases of implantoplasty or curetting the implant. Nonetheless, when considering the available scientific data, it appears such results might be even more positive and more sustainable over the longer term if mechanical abrasive treatment were replaced by mechanical non-contact or mechanical contact non-abrasive treatments.
III. A treatment plan, according to the EFP clinical practice guidelines
By Mia Rakic
Based on the available information (from the picture and case description), including relatively stable reduced periodontium at adjacent teeth and pronounced bone resorption buccally, without suppuration, I would conclude that the buccal position of the implant played a role in this case of peri-implantitis.
Following detailed periodontal prophylaxis/treatment, I would opt for non-surgical treatment to maximally reduce infection and inflammation and then I would continue with surgical treatment given the morphology and dimension of the peri-implant crater.
My surgical treatment would include degranulation using titanium, plastic, or graphite curettes, followed by surface decontamination with an ultrasonic device (with titanium tips) and titanium brushes (Ramanauskaite et al., 2023). Surface decontamination should be followed by abundant rinsing with sterile saline for maximal removal of bacterial biofilms, cement remnants, particles, and degenerated tissue. According to the EFP guideline, implantoplasty, photodynamic therapy, local antimicrobials, and chlorhexidine are not recommended for implant-surface decontamination (Wilensky et al., 2023), but I would perhaps rub the implant with gauze soaked with some standard solutions such as hydrogen peroxide or iodide.
The EFP guideline recommends reconstructive procedures for intra-osseous defects with a depth of ≥3mm, so I would opt for guided bone regeneration. The buccally oriented defect additionally determines my decision to use a bone substitute with membrane to regenerate and reinforce the buccal site of the implant.
Information about the peri-implant phenotype is not available, but it is something that needs to be assessed, as lack of an adequate width of keratinized mucosa is associated with an increased prevalence of peri-implantitis. The patient would be scheduled for frequent visits during the first year and included in a strict maintenance regimen.
References
Abedi A, Formanek B, Russell N, Vizesi F, Boden SD, Wang JC, Buser Z (2020). Examination of the role of cells in commercially available cellular allografts in spine fusion: An in vivo animal study. J Bone Joint Surg Am. 102:e135, 1-10.
Abrahammson I & Cardaropoli G (2007). Peri-implant hard and soft tissue integration to dental implants made of titanium and gold. Clin Oral Implants Res. 18(3), 269-74.
Albrektsson T, Tengvall P, Amengual L, Coli P, Kotsakis GA, Cochran D (2023). Osteoimmune regulation underlies oral implant integration and its perturbation. Front Immunol. 13:1056914. doi: 10.3389/fimmu.2022.1056914.
Cho-Ying Lin et al. (2022). The impact of implantoplasty in regenerated and non-regenerated treatment modalities in peri-implantitis: a systematic review and meta-Analysis. Int J Oral Maxillofac Implants. 37, 859-868.
Cooke JW, Sarment DP, Whitesman LA, Miller SE, Jin Q, Lynch SE, Giannobile WV (2006). Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 12(6),1441-50.
Delgado-Ruiz R & Romanos G (2018). Potential causes of titanium particle and ion release in implant dentistry: a systematic review. Int J Mol Sci. 2018. 19(11), 3585. Doi:10.3390/ijms19113585.
Eger M, Hiram-Bab S, Liron T, Sterer N, Carmi Y, Kohavi D, Gabet Y (2018). Mechanism and prevention of titanium particle-induced inflammation and osteolysis. Front Immunol. 9, 2963. Doi:10.3389/fimmu.2018.02963. PMID:30619321; PMCID:PMC6305459.
Eger, M., Sterer, N., Liron, T., Kohavi, D. and Gabet, Y., 2017. Scaling of titanium implants entrains inflammation-induced osteolysis. Scientific Reports, 7(1), p.39612.
El Chaar E, Almogahwi M, Abdalkader K, Alshehri A, Cruz S, Ricci J (2020). Decontamination of the Infected Implant Surface: A Scanning Electron Microscope Study. Int J Periodontics Restorative Dent. 40, 395-401.
Esposito M, Grusovin MG, Worthington HV (2012). Treatment of peri-implantitis: What interventions are effective? A Cochrane systematic review. Eur J Oral Implantol. 5(suppl): S21-S41. PMID:22834392.
Fletcher P, Deluiz D, Tinoco EM, Ricci JL, Tarnow DP, Tinoco JM (2017). Human histologic evidence of reosseointegration around an implant affected with peri-implantitis following decontamination with sterile saline and antiseptics: A case history report. Int J Periodontics Restorative Dent. 37(4):499-508.
Froum SJ, Froum SH, Rosen PS (2012). Successful management of peri-implantitis with a regenerative approach: A consecutive series of 51 treated implants with 3-to-7.5-year follow-up. Int J Periodontics Restorative Dent. 32,11-20.
Froum SJ, Froum SH, Rosen PS (2015). A regenerative approach to the successful treatment of peri-implantitis: A consecutive series of 170 implants in 100 patients with 2–10-year follow-up. Int J Periodontics Restorative Dent. 35, 857-863.
Froum SJ & Rosen PS (2014). Re-entry evaluation following treatment of peri-implantitis with a regenerative approach. Int J Periodontics Restorative Dent. 34(1), 47-59.
Gomes PDS, Daugela P, Poskevicius L, Mariano L, Fernandes MH (2019). Molecular and cellular aspects of socket healing in the absence and presence of graft materials and autologous platelet concentrates: A focused review. J Oral Maxillofac Res. 10:e2. doi: 10.5037/jomr.2019.10302.
Grunder U, Gracis S, Capelli M (2005). Influence of the 3D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent. 25(2), 113-9.
Hermann JC, Jones AA, Bakaeen LG, et al. (2011). Influence of a machined collar on crestal bone changes around titanium implants: a histometric study in the canine mandible. J Periodontol. 82(9), 1329-38.
Herrera D, Berglundh T, Schwarz F, Chapple I, Jepsen S, Sculean A, Kebschull M., Papapanou PN, Tonetti MS, Sanz M and EFP workshop participants and methodological consultant (2023). Prevention and treatment of peri‐implant diseases—The EFP S3 level clinical practice guideline. Journal of Clinical Periodontology, 50 (S26), 4-76.
Htet M, Madi M, Zakaria O, et al. (2016) Decontamination of anodized implant surface with different modalities for peri-implantitis treatment: Lasers and mechanical debridement with citric acid. J Periodontol. 87, 953-961.
Imamura K, Hamada Y, Yoshida W, Murakami T, Nakane-Koyachi S, Yoshikawa K, Saito A (2022). Investigating the effects of dehydrated human amnion-chorion membrane on periodontal healing. Biomolecules. 12(6), 857.
Isehed C, Svenson B, Lundberg P, Holmlund A (2018). Surgical treatment of peri-implantitis using enamel matrix derivative, an RCT: 3- and 5-year follow-up. Journal of Clinical Periodontology 45(6):744-753
Karoussis IK, Kyriakidou K, Papaparaskevas J, Vrotsos IA, Simopoulou M, Kotsakis GA (2018). Osteostimulative calcium phosphosilicate biomaterials partially restore the cytocompatibility of decontaminated titanium surfaces in a peri-implantitis model. J Biomed Mater Res B Appl Biomater, 106(7):2645-2652
Kister F, Specht O, Warkentin M., Geis-Gerstorfer J, Rupp, F (2017). Peri-implantitis cleaning instrumentation influences the integrity of photoactive nanocoatings. Dental Materials, 33(2), e69-e78.
Kotsakis GA & Ganesan SM (2025). Microbial dysbiosis, titanium release, and peri-implantitis. Journal of Dental Research, 104(5), 473-480. doi: 10.1177/00220345241307939.
Kotsakis GA & Olmedo DG (2021). Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontology 2000 86, 231-240.
Kotsakis GA, Lan C, Barbosa J, Lill K, Chen R, Rudney J, Aparicio C (2016). Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J Periodontol. 87(7):809-19
Kotsakis GA, Xie L, Siddiqui DA, Daubert D, Graham DJ, Gil FJ (2024). Dynamic assessment of titanium surface oxides following mechanical damage reveals only partial passivation under inflammatory conditions. Npj Mater Degrad. 8(1):98. doi: 10.1038/s41529-024-00514-1
Rakic M, Radunovic M, Petkovic‐Curcin A, Tatic, Z, Basta‐Jovanovic G, Sanz M (2022) Study on the immunopathological effect of titanium particles in peri‐implantitis granulation tissue: A case–control study. Clinical Oral Implants Research, 33(6), 656-666.
Lallier T, Yukna R, St. Marie S, Moses R (2001). Putative collagen binding protein hastens attachment. Journal Periodontology. 72, 990-997.
Lin C-Y, Chen C, Chiang H-L, Pan W-L, Wang H-L (2022). The Impact of implantoplasty in regenerated and nonregenerated treatment modalities in peri-implantitis: A systematic review and meta-analysis. Int J Oral Maxillofac Implants. 37(5), 859-868.
Miron RJ, Sculean A, Cochran D, Froum S, Zucchelli G, Nemcovsky C, et al. (2016). Twenty years of enamel matrix derivative: the past, the present and the future. Journal of Clinical Periodontology 43(8), 668-683. doi: 10.1111/jcpe.12546.
Monje A, Chappuis V, Monje F, Muñoz F, Wang HL, Urban IA, Buser D (2019). The critical peri-implant buccal bone wall thickness revisited: An experimental study in the beagle dog. Int J Oral Maxillofac Implants. 34(6),1328–1336.
Qian S-J, Tsai, Y-W, Koutouzis T, Lai H-C, Qiao S-C, Kotsakis GA (2024). Impact of surface chemical treatment in surgical regenerative treatment of ligature-induced peri-implantitis: A canine study. Journal of Periodontology. 95(10):991-1001. doi: 10.1002/JPER.23-0634.
Ramanauskaite A, Schwarz F, Cafferata EA, Sahrmann P (2023). Photo/mechanical and physical implant surface decontamination approaches in conjunction with surgical peri‐implantitis treatment: a systematic review. Journalof Clinical Periodontology, 50 (S26), 317-335.
Rosen PS, Froum SJ, Sarmiento HL, Wadhwani CPK (2022). A revised peri-implantitis classification scheme: Adding three-dimensional considerations to facilitate prognosis and treatment planning. Int J Periodontics Restorative Dent. 42, 291-299.
Rosen PS, Qari M, Froum SJ, Dibart S, Chou, LL (2018). A pilot study on the efficacy of a treatment algorithm to detoxify dental implant surfaces affected by peri-implantitis. Int J Periodontics Restorative Dent. 38, 261-268.
Rosen PS & Tarnow DP (2023). Subcrestal implantoplasty for treating peri-implantitis with regenerative care- How deep should treatment go? Compend Contin Educ Dentistry. 44(8), 440-446.
Salle MR, Deluiz D, Fletcher P, Santoro M, Tinoco E (2023). Decontamination and repair protocol promotes positive outcomes in implants affected by peri-implantitis: A human case series. Int J Periodontics Restorative Dent. 43, 699-705. doi: 10.11607/prd.5534.
Schiff N, Grosgogeat B, Lissac M, Dalard F (2022). Influence of fluoride content and pH on the corrosion resistance of titanium and its alloys. Biomaterials. 23(9),1995–2002. Doi: 10.1016/s0142-9612(01)00328-3. PMID: 11996041.
Schmage P, Thielemann J, Nergiz I, Scorziello T, Pfeiffer P (2012). Effects of 10 cleaning instruments on four different implant surfaces. Int J Oral Maxillofac Implants. 27, 308-317. PMID:22442769.
Schwarz F, Jepsen S, Herten M, Sager M, Rothamel D, Becker J (2006) Influence of different treatment approaches on non-submerged and submerged healing of ligature induced peri-implantitis lesions: an experimental study in dogs. Journal of Clinical Periodontology 33(8), 584-595.
Schwarz F, Herten M, Sager M, Bieling K, Sculean A, Becker J (2007). Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin Oral Implants Res. 18(2),161-70.
Slots J (2012). Low-cost periodontal therapy. Periodontology 2000. 60(1),110-37. Doi: 10.111/j.1600-0757.2011.00429.x. PMID:22909110.
Verdeguer P, Gil J, Punset M, Manero JM, Nart J, Vilarrasa J, Ruperez E (2022). Citric acid in the passivation of titanium dental implants: Corrosion resistance and bactericide behavior. Materials. 15(2), 545.
Wachi T, Shuto T, Shinohara Y, Matono Y, Makihira S (2015). Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology, 327, pp.1-9.
Walivaara B, Lundstrom I, Tengvall P (1993). An in-vitro study of H2O2 treated titanium surfaces in contact with blood plasma and a simulated body fluid. Clin Mater. 12, 141-148. doi: 10.1016/0267-6605(93)90065-f. PMID: 10148559.
Wilensky A, Shapira L, Limones A, Martin C (2023). The efficacy of implant surface decontamination using chemicals during surgical treatment of peri‐implantitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 50 (S26), 336-358.
The panel

Georgios Kotsakis, Professor & Assistant Dean for Clinical Research, Department of Oral Biology & Clinical Research Center, Rutgers School of Dental Medicine, Newark, NJ, USA.
Paul Fletcher, Associate Clinical Professor, Department of Periodontics and Endodontics, Stony Brook University School of Dental Medicine, Stony Brook, NY, USA.
Paul Rosen, Clinical Professor of Periodontics, Rutgers University School of Dental Medicine, Newark, NJ, USA.
Dennis Tarnow, Clinical Professor, Columbia College of Dental Medicine, New York, NY, USA.
Mia Rakic, Associate Professor, Department of Periodontology, ZMK, University of Bern, Switzerland.




