Research, Clinical & Translational Research, Article
Development of in-vitro multispecies subgingival biofilm models
14 March 2023
The development of a dynamic in vitro model with multispecies biofilm is helping increase our undertstanding of the behaviour of subgingival bacteria within biofilms and assess the impact of different implant surfaces on biofilm growth, as well as the efficacy of differerent implant-surface decontamination methods. In the first of a series of articles for Perio Insight on the research carried out at the universities that teach the EFP-accredited programme in periodontology, Honorato Ribeiro-Vidal outlines work being conducted by his research group at the University Complutense of Madrid (Spain).
Bacterial biofilms can be defined as complex, functionally and structurally organized sessile microbial communities, characterised by a multi-species diversity that synthesises an extracellular, biologically active polymer matrix – exopolysaccharides (EPS) – which anchors cells to each other as well as to surfaces. These microbial communities may be found in a wide variety of biotic and abiotic surfaces, including living tissues, such as teeth, and medical devices, such as dental implants.
In fact, periodontal and peri-implant diseases are related to the formation and maturation of subgingival biofilms, which not only serve as a reservoir and source of pathogens but can also prevent effective antimicrobial therapeutic strategies. To improve knowledge about the structure and pathogenicity of these subgingival biofilms, different research models have been developed over the years, from the direct observation of naturally occurring biofilms on oral surfaces to the in vitro generation of properly standardised multispecies biofilm models.
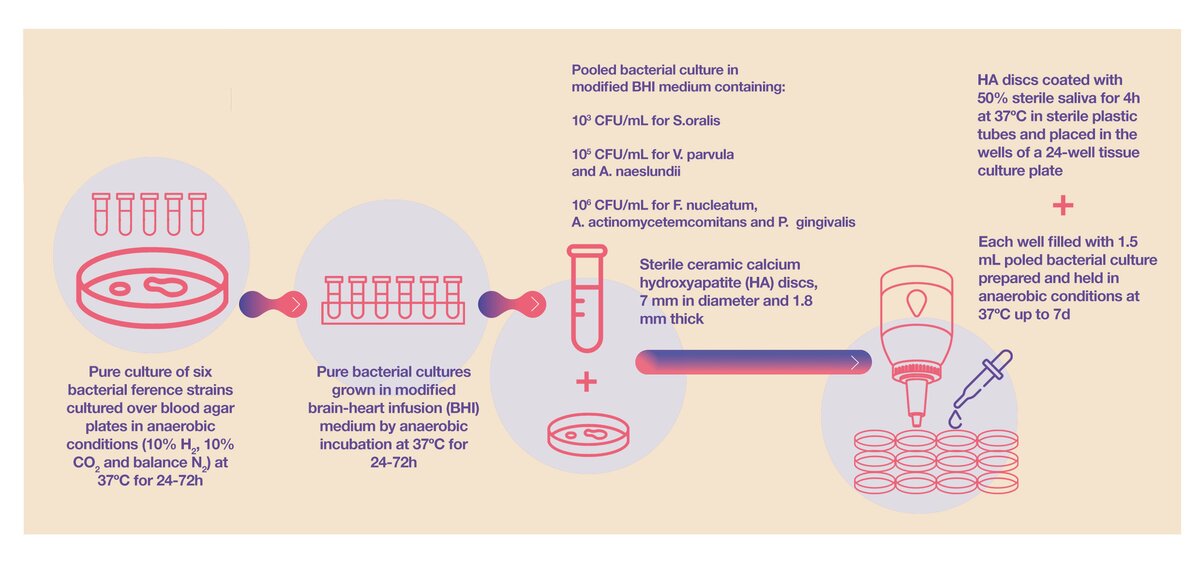
The ETEP (Aetiology and Therapy of Periodontal and Peri-Implant Diseases) research group at the University Complutense of Madrid (Spain) has been active in this line of research for over a decade. In 2011, in collaboration with the Dentaid Research Center, the first paper on the development of an in vitro subgingival biofilm static model was published (Sanchez et al., 2011). This model included six reference bacterial strains (see Figure 1) that follow a pattern of bacterial colonisation and maturation similar to the biofilms encountered in in vivo subgingival environments:
- initial colonisation: Streptococcus oralis and Actinomyces naeslundii.
- early colonisation: Veillonella parvula.
- secondary colonisation: Fusobacterium nucleatum,
- late colonisation: Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans.
This static model allowed us to study the impact of different antibacterial compounds on biofilm formation. We studied the impact of commonly used antiseptic agents – for example, chlorhexidine and cationic quaternary ammonium compounds such as cetylpyridinium chloride (CPC) (Sanchez et al., 2017) – as well as the impact of natural ingredients such as wine extracts or omega-3 fatty acids (Ribeiro-Vidal et al., 2020).
The biofilm static model was later modified to study biofilm formation on different surfaces where, instead of using discs of these different surfaces (hydroxyapatite, titanium, or zirconia), the model was modified to grow the biofilms directly on the implants, which allowed us to study the relevance of the implant macro-surface topography on the biofilm formation (Figure 2) (Bermejo et al., 2019).
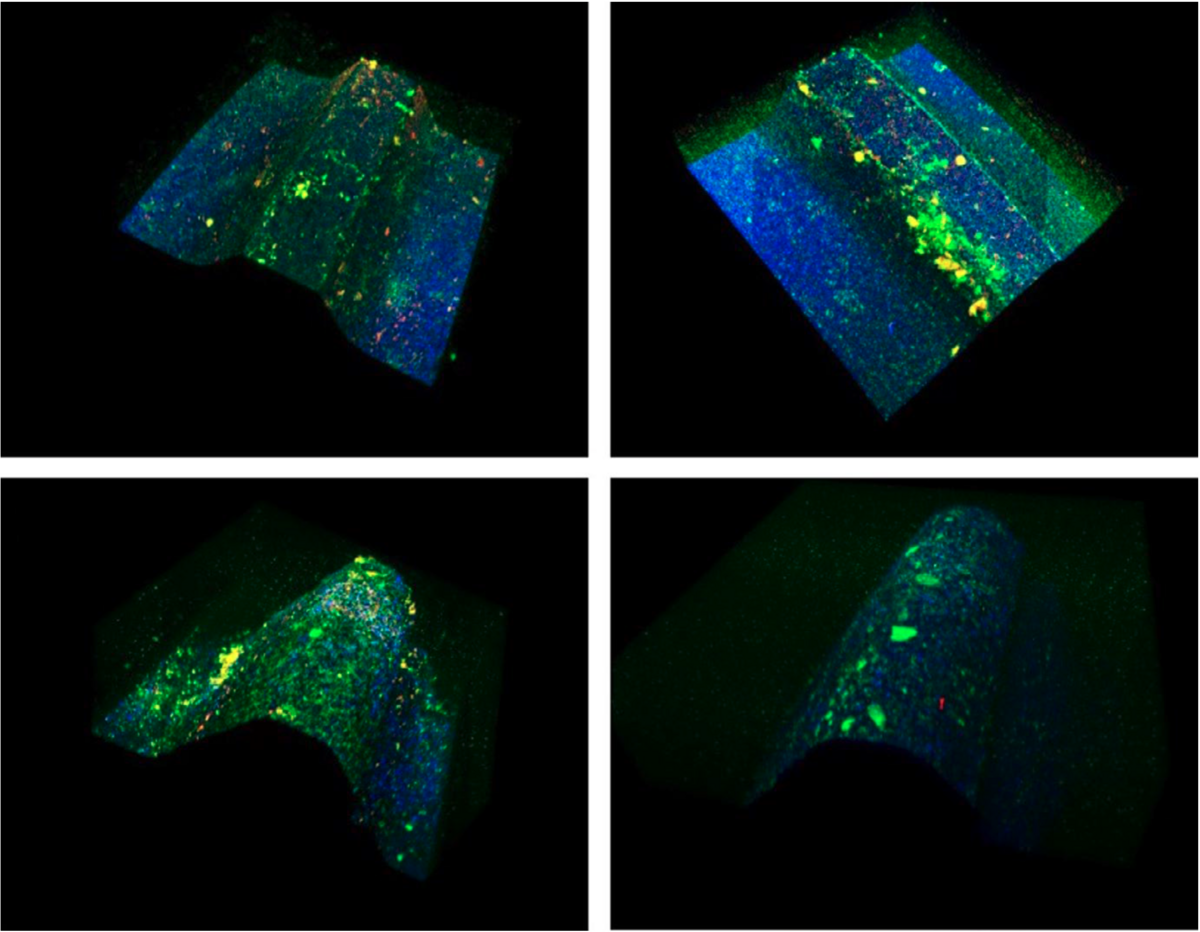
Using LIVE/DEAD BacLight fluorescence, it is possible to differentiate live bacteria (in green), dead bacteria (in red), and the implant surface (in blue). Microcolonies are mostly concentrated and completely cover the lateral surfaces and the peak of the threads, while the area between threads depicts mostly implant material.
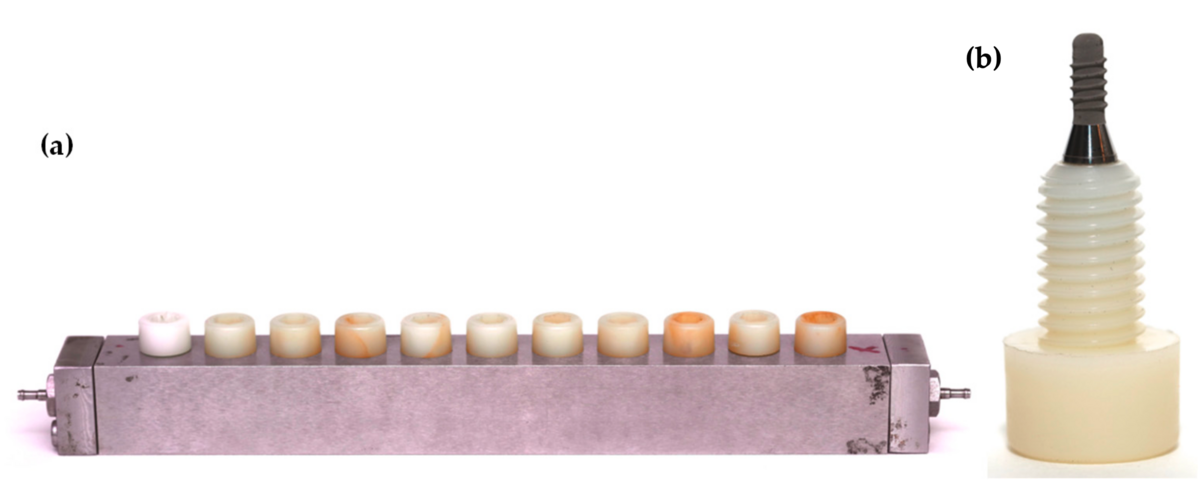
But these in vitro static biofilm models lack the environmental conditions that naturally occur in the mouth because they do not control for temperature, pH, and oxygen reduction (PO2). Furthermore, they do not incorporate the flow and shear forces that are exerted by the saliva and crevicular fluid when biofilm is growing in the oral cavity. To overcome these problems and to improve the translation ability of the model, we developed a dynamic in vitro multispecies biofilm model using a bioreactor and a Robbins device, which fosters the growth of subgingival bacteria that are exposed to environmental conditions similar to the oral cavity (Blanc et al, 2014) (Figure 3).

(a) culture medium, brain heart infusion (BHI); (b) peristaltic pumps; (c) incubation recipient; (d) bioreactor (temperature control, pH, pO2, agitation, and weight); (e) Robbins device hosting the developing biofilms.
This dynamic subgingival biofilm model was later modified to hold dental implants rather than disks by using a modified Robbins device that allowed the formation and maturation of the biofilms directly on the implant surfaces, reproducing the environmental conditions of the oral cavity (Sanchez et al, 2021) (Figure 4).
With this modification, our research group is currently studying the differential implant micro-surface topography on biofilm growth. Preliminary data using both scanning electron microscopy (SEM) and confocal laser microscopy (CLSM) clearly show that moderately rough titanium surfaces accumulate a greater quantity and complexity of biofilms when compared to smooth titanium surfaces – a finding that may have clear clinical implications (Figure 5).
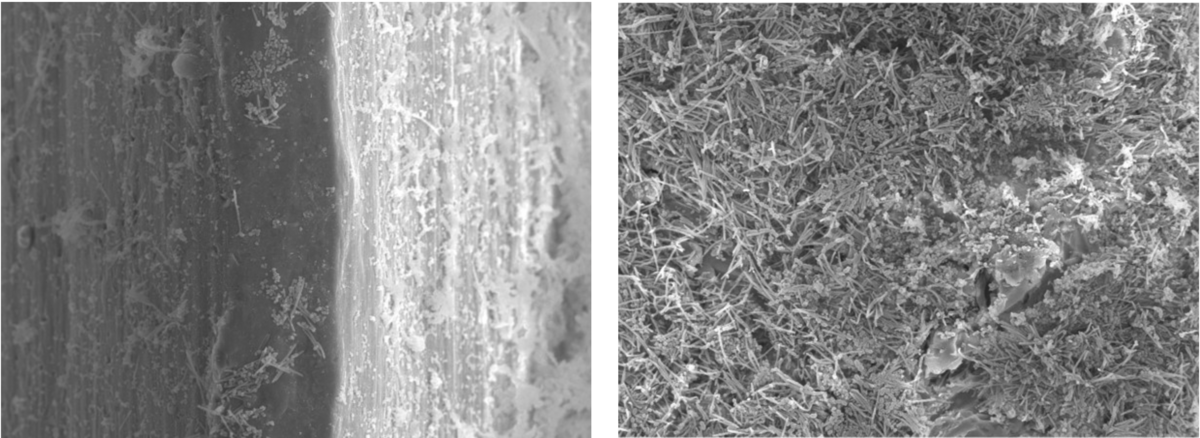
In conclusion, the biofilm research being carried out at the Complutense University of Madrid has shown a great potential to study the complex interactions of the bacteria within the biofilm and the impact on the biofilm growth of the surfaces and different antimicrobial strategies. This is particularly relevant in the study of biofilm-implant surface interactions in light of the current clinical relevance of peri-implant diseases.
This in vitro research model provides an additional step in generating knowledge of oral biofilms and translating the possible impact of surface microtopography on their growth and elimination through different decontamination methods.
Select bibliography
Bermejo, P., Sanchez, M. C., Llama-Palacios, A., Figuero, E., Herrera, D., & Sanz, M. (2019). Topographic characterization of multispecies biofilms growing on dental implant surfaces: An in-vitro model. Clin Oral Implants Res, 30(3), 229-241. doi:10.1111/clr.13409
Blanc, V., Isabal, S., Sanchez, M. C., Llama-Palacios, A., Herrera, D., Sanz, M., & Leon, R. (2014). Characterization and application of a flow system for in vitro multispecies oral biofilm formation. J Periodontal Res, 49(3), 323-332. doi:10.1111/jre.12110
Ribeiro-Vidal, H., Sanchez, M.C., Alonso-Espanol, A., Figuero, E., Ciudad, M.J., Collado, L., Herrera, D., Sanz, M. Antimicrobial Activity of EPA and DHA against Oral Pathogenic Bacteria Using an In Vitro Multi-Species Subgingival Biofilm Model. Nutrients 2020, 12, 2812.
Sanchez, M. C., Llama-Palacios, A., Blanc, V., Leon, R., Herrera, D., & Sanz, M. (2011). Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota. J Periodontal Res, 46(2), 252-260. doi:10.1111/j.1600-0765.2010.01341.x
Sanchez, M.C., Fernandez, E., Llama-Palacios, A., Figuero, E., Herrera, D., Sanz, M. (2017) Response to antiseptic agents of periodontal pathogens in in vitro biofilms on titanium and zirconium surfaces. Dent. Mater. 2017, 33, 446–453.
Sanchez, M.C., Alonso-Español, A., Ribeiro-Vidal, H., Alonso, B., Herrera, D., Sanz, M. (2021). Relevance of Biofilm Models in Periodontal Research: From Static to Dynamic Systems. Microorganisms 9, 428. doi.org/10.3390/microorganisms9020428

Biography
Honorato Ribeiro-Vidal received his dental degree from the Portuguese Catholic University (Viseu, Portugal) in 2012 and was awarded the degrees of Master of Science and “Expert in clinical periodontology” by the Complutense University of Madrid (Madrid, Spain) in 2014. In 2018, he completed his three-year postgraduate speciality training in periodontology and implant dentistry at the Complutense University of Madrid, accredited by the EFP, and in 2020 he finished the PhD programme at the same university. He is currently a guest assistant professor at the Faculty of Dental Medicine of Oporto University and also runs a private practice in Aveiro, Portugal.
Honorato Ribeiro-Vidal is the lead author of this report. The other researchers involved were Maria Sánchez, Andrea Alonso-Español, Elena Figuero, David Herrera, and Mariano Sanz. All are members of the ETEP Research Group at the University Complutense of Madrid (Spain).