Prevention & Public health, Therapy
The role of micronutrients in periodontal disease prevention and therapy
21 June 2023
Vitamins, minerals, and trace elements can help prevent periodontal disease and be effective adjunctives to periodontal therapy.
Diets such as the Mediterranean Diet and the Okinawan-based Nordic Diet can reduce gingival inflammation, while the “western” diet is proinflammatory.
More research is needed to support these initial positive findings of these host-modulating approaches.
The role of nutrition in periodontal health has recently come to the fore as research has increasingly explored host-modulation techniques as a way to prevent disease and as an adjunctive to periodontal therapy. Denica Kuzmanova and Henrik Dommisch describe the current understanding of the impact of diet and of vitamins and other micronutrients on preventing and treating periodontitis.
Current periodontal standard-of-care measures are aimed at preventing and controlling periodontitis and establishing or maintaining balanced interactions between microbial factors and the immune-inflammatory host response that are compatible with periodontal health. Although effective in the majority of patients with periodontitis, these interventions have their limitations. They may cause side effects or result in only partial restoration of health, leaving features of biofilm dysbiosis and inflammation.
In addition, research has revealed that in susceptible individuals, periodontal tissue damage is predominantly mediated by the dysregulated host inflammatory response to the subgingival microbial challenge. This leads to an environment that favours inflammation and contributes to the exacerbation of the microbiota imbalance, inflammation, and overt periodontitis.
With the goal of restraining inflammation to control infection, it has been suggested that host-response modulation strategies could be promising adjunctive treatments to conventional periodontal therapy. In fact, the link between diet and oral health has long been acknowledged. In 2011, the EFP’s European Workshop on Periodontology (Perio Workshop) found emerging evidence that the nutritional modulation of periodontal inflammation was one such promising approach.
Micronutrients
Micronutrients are vital substances, even though they are needed in smaller quantities (mg/µg) compared to macronutrients (proteins, carbohydrates, and fats). The group includes water- and fat-soluble vitamins, minerals, and trace elements that are needed for a variety of metabolic and physiologic processes, the regulation of inflammation and immunity, and for growth and development (Dommisch et al. 2018). They are essential, given that for the most part they cannot be produced by humans and must be obtained from food.
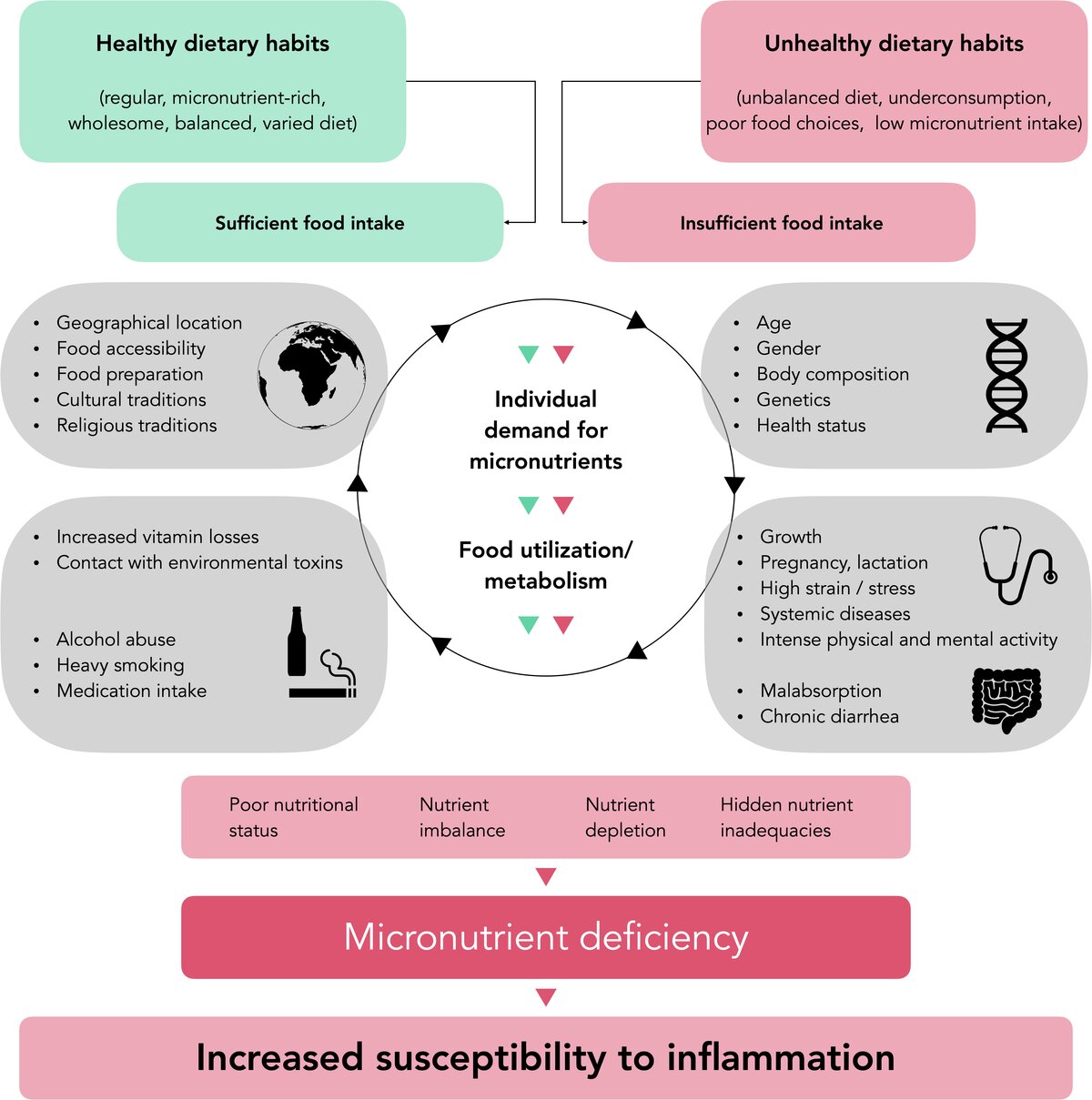
Very low dietary intake of vitamins and minerals can result in deficiency disease or inadequacies, which are a health problem not confined to low- and middle-income countries. This "hidden hunger" can be caused either by prolonged underconsumption or by poor food choices. Both undernutrition and obesity can coexist with selected micronutrient deficiencies. In people with adequate dietary intake, such deficiencies may occur during periods of increased individual micronutrient requirements, but also in conditions of increased loss of micronutrients, such as alcohol abuse and heavy smoking (Figure 1). Malnutrition and insufficient oral hygiene are two important factors that predispose to necrotising gingivitis. One systematic analysis concluded that micronutrient deficiencies, such as for vitamin C, vitamin D or vitamin B12, may be associated with the onset and progression of periodontal diseases (Chapple et al. 2017).
Vitamins
Vitamin C (ascorbic acid, AA) plays an important role in collagen synthesis, helps maintain the structural integrity of connective tissue, and has a protective effect on maintaining tissue homeostasis. It is a water-soluble antioxidant that neutralizes oxidative stress and is depleted in this process. AA deficiency has been considered a systemic risk factor among diet-related diseases that can modify the immuno-inflammatory response and has been included in the current (2018) classification of periodontal diseases agreed by the EFP and the American Academy of Periodontology.
Cross-sectional studies have found lower serum/plasma AA concentrations in patients with gingivitis or periodontitis, and in smokers, than in periodontally healthy individuals and non-smokers. An inverse relationship has been reported between plasma AA levels and the degree of periodontal inflammation as well as severity in different populations and age groups (Van der Velden et al. 2011). Furthermore, total antioxidant capacity has been associated with periodontitis while elevated serum antioxidant levels have reduced relative disease risk. Case-control studies have shown that periodontitis patients had lower AA plasma levels, lower AA intake, more bone loss, and greater periodontal disease progression than the healthy controls (Kuzmanova et al. 2012).
Other studies have shown that, while AA depletion resulted in gingival bleeding regardless of the level of individual oral hygiene, both the consumption of AA-rich fruits and AA supplementation had an inhibitory effect on sulcular bleeding in non-smoking patients with gingivitis and chronic periodontitis. However, this AA consumption or supplementation was less effective following periodontal nonsurgical interventions (Tada et al. 2019).
There are indications that the function of AA is superior when it is obtained from fruits rather than from dietary supplements. Fruits provide several additional micronutrients, phytochemicals, and dietary fibres that may influence the bioavailability of AA and have other beneficial effects.

Vitamin E comprises of a set of lipophilic antioxidant food compounds termed as α, β, γ, and δ tocopherols or tocotrienols. Their primary function, to protect cell membranes from reactive oxygen species and to regulate immune responses, has been widely studied (Shadisvaaran et al. 2021). While some epidemiological studies have observed an association between serum vitamin E concentration and periodontitis, others have not. Prospective studies have shown that higher vitamin E intake was associated with a lower number of teeth affected by periodontitis. Conversely, low serum alpha-tocopherol levels were associated with periodontal progression.
Intervention studies have suggested a positive effect of higher alpha-tocopherol intake on periodontal healing after subgingival instrumentation (scaling and root planing). The adjunctive intake of vitamin E has been shown to have significant beneficial effects on periodontal parameters as well as antioxidant defence compared to controls. Current literature suggests that vitamin E could improve periodontal status by correcting redox status imbalance, reducing inflammatory responses, and promoting wound healing. However, evidence from clinical trials is still limited.
Vitamin D is a fat-soluble micronutrient found only in small amounts in food and is a secosteroid hormone produced mainly by the skin when exposed to sunlight. Vitamin D plays an important role in calcium and bone metabolism. It is also thought to have immunomodulatory and anti-inflammatory effects. In patients with periodontitis, lower vitamin D levels compared with healthy controls have been reported. Higher serum 25-hydroxy vitamin D (25OHD) concentrations have been associated with lower rates of gingivitis and less attachment and tooth loss.
One prospective study reported that every 10 μL/L increase in serum 25OHD was associated with a 13% decrease in tooth loss, while another showed that Vitamin D supplementation after nonsurgical periodontal therapy resulted in a slight reduction in probing pocket depth (PPD) and attachment loss compared to placebo controls and improved anti-inflammatory response. Well-designed prospective and intervention studies are needed to define what plasma vitamin D concentration is required prior to the initiation of periodontal treatment to achieve the best therapeutic outcome.
The water-soluble B vitamins are involved in multiple processes, including metabolism, erythrocyte production, and collagen synthesis and they act as coenzymes in several enzymatic processes that support every aspect of cellular physiological functioning. Epidemiological data have demonstrated a higher severity of periodontal disease in individuals with inadequate dietary intake or serum levels of vitamin B9 (folate). Among patients with periodontal disease, smokers had lower serum folic acid concentrations than non-smokers. In a prospective cohort study, an increase in serum vitamin B12 was linked to a decrease in clinical periodontal parameters and tooth loss.
Giving a vitamin B-complex preparation to periodontitis patients after periodontal surgery has improved clinical attachment levels compared with patients receiving placebo. Furthermore, adjunctive systemic folate intake after subgingival instrumentation has resulted in significant additional gain in clinical attachment in patients with stage II-III periodontitis. Nevertheless, further clinical and biochemical data are needed to support these findings.
Vitamin A is a group of different lipid-soluble compounds (retinol, retinal, retinoic acid, provitamin A carotenoids) that play a crucial role in physiological processes such as cellular growth and differentiation, immune-system functioning, bone and foetus development, vision, and the formation of the central nervous system. Provitamin A carotenoids also act as antioxidants. Large-scale epidemiological studies have found either a weak or no association between vitamin A inadequacy and periodontitis and its efficacy in managing periodontitis progression is unclear (Dommisch et al. 2018). In contrast, higher dietary intake of β-carotene has been associated with a significantly lower percentage of sites with PPD >3mm after non-surgical treatment and a greater reduction in PPD in non-smokers than in smokers with periodontitis. Low serum carotenoid levels (α-, β-carotene, β-cryptoxanthin) have been associated with a significantly increased risk of periodontitis. In addition, a weak inverse relationship has been reported between the prevalence of mild periodontitis and serum concentrations of α-carotene, β-carotene and β-cryptoxanthin.
Lycopene, a pigment derived from red fruits, has been suggested to be one of the most effective in vitro singlet oxygen quenchers of the carotenoids. Research has shown that individuals with higher serum lycopene concentrations had reduced C-reactive protein (CRP) levels, while those with higher tomato consumption showed reduced leukocyte counts. An association between chronic periodontitis and an increased risk of heart failure has been suggested, with higher monthly tomato consumption reducing this risk in periodontitis patients. Intervention studies with a combined preparation of lycopene and other micronutrients (vitamins A, C, E, selenium, zinc) for oral application adjunctive to mechanical debridement have shown positive effects on periodontal healing.
Minerals and trace elements
Significant inverse relationships have been reported between serum/plasma levels of calcium, magnesium, zinc, and manganese and periodontal severity/progression. Studies have shown that a low serum calcium-magnesium ratio is significantly associated with increased attachment loss and the progression of periodontal disease, and that individuals with periodontitis have a significantly lower dietary intake of calcium, magnesium, copper, selenium, and antioxidant nutrients – and respectively lower serum and saliva levels – compared to controls.
It has been suggested that milk and milk products – a source of calcium, phosphate, and various proteins – may have beneficial effects on periodontal health while, conversely, dietary calcium intakes below recommended reference levels have been associated with increased risk of tooth loss, severity of periodontal disease, and attachment loss.
Case-control studies have revealed significantly lower magnesium, selenium, and zinc levels in diabetic and non-diabetic patients with periodontitis compared to healthy controls. Other studies have shown non-surgical periodontal therapy leading to increased serum zinc levels in Type-2 diabetes mellitus patients with periodontitis, while subjects with magnesium supplementation have lower attachment loss and higher tooth retention compared to non-supplemented control subjects.
The current evidence on nutraceutical and food-based interventions as an adjunct to non-surgical periodontal therapy has been recently reviewed in a systematic review by Johan Peter Woelber and colleagues at the universities of Freiburg and Heidelberg in Germany (Woelber et al. 2023).
Diet and prevention
The prevention of non-communicable diseases (NCD) focuses on dietary changes to reduce the systemic inflammatory burden. A Western diet – rich in refined grains, red meat, high-fat dairy products, simple carbohydrates, and consumption of processed food – has been described as pro-inflammatory.
Cross-sectional data have indicated that a “high-quality” diet is identified as a health-promoting factor along with control of normal weight and adequate exercise. Participants who adhered to these three factors have shown a 40% lower risk of developing periodontitis. Nonetheless, the role of physical activity, weight loss, and dietary counselling is the focus of further research.
A pro-inflammatory diet and poor micronutrient intake have been linked to an increased risk of periodontal disease. In contrast, adherence to an anti-inflammatory dietary pattern “high micronutrient and fibre” has been linked to a lower risk of periodontitis and tooth loss.
Dietary anti-inflammatory interventions such as Mediterranean diet, the Okinawan-based Nordic Diet (OBND), and so-called Palaeolithic diets have resulted in reduced gingival inflammation despite constant – or even increasing – plaque accumulation. In patients with periodontitis and either metabolic syndrome or Type-2 diabetes mellitus, dietary change to a wholesome nutrition or OBND led to improvements in both the periodontal and systemic inflammatory parameters without providing professional oral hygiene. Fasting has also been shown to facilitate the reduction of periodontal inflammation.
The need for more research
In conclusion, there is increasing evidence of the overall importance of micronutrients on general and periodontal health. Furthermore, the dietary source of micronutrients needs to be considered in the context of favourable dietary patterns based on a wide variety of micronutrient-rich foods as well as putative interactions between various micronutrients. Future research is required to further elucidate the role of micronutrients in the prevention and treatment of periodontal disease. Prospective and clinical intervention studies may help to define causal relationships, potential intervention strategies, and future recommendations.
While this article reflects some of the important aspects regarding the role of micronutrients in the prevention of periodontal disease and in periodontal therapy, it does not claim to offer a complete picture, since putative relevant aspects – such as genetics, microbiology, micronutrient deficiencies, and adverse effects – could not be covered extensively .
Select bibliography
Iain L C Chapple, Philippe Bouchard, Maria Grazia Cagetti, Guglielmo Campus, Maria-Clotilde Carra, Fabio Cocco, Luigi Nibali, Philippe Hujoel, Marja L Laine, Peter Lingstrom, David J Manton, Eduardo Montero, Nigel Pitts, Hélène Rangé, Nadine Schlueter, Wim Teughels, Svante Twetman, Cor Van Loveren, Fridus Van der Weijden, Alexandre R Vieira, Andreas G Schulte. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017 Mar;44 Suppl 18:S39-S51. doi: 10.1111/jcpe.12685. PMID: 28266114.
Henrik Dommisch, Denica Kuzmanova, Daniel Jönsson, Melisa Grant, Iain Chapple. Effect of Micronutrient Malnutrition on Periodontal Disease and Periodontal Therapy. Periodontology 2000. 2018;78:129–153. doi: 10.1111/prd.12233.
Denica Kuzmanova, Ineke DC Jansen, Ton Schoenmaker, Kamran Nazmi, Wijnand J Teeuw, Sergio Bizzarro, Bruno G Loos, Ubele van der Velden. Vitamin C in plasma and leucocytes in relation to periodontitis. J Clin Periodontol. 2012 Oct;39(10):905-12. doi: 10.1111/j.1600-051X.2012.01927.x.
An Li, Yuntao Chen, Annemarie A Schuller, Luc VM van der Sluis, Geerten-Has E Tjakkes. Dietary inflammatory potential is associated with poor periodontal health: A population-based study. J Clin Periodontol. 2021 Jul;48(7):907-918. doi: 10.1111/jcpe.13472.
Priscilla Martinon, Laurie Fraticelli, Agnes Giboreau, Claude Dussart, Denis Bourgeois, Florence Carrouel. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J Clin Med. 2021 Jan 7;10(2):197. doi: 10.3390/jcm10020197.
Saminathan Shadisvaaran, Kok Yong Chin, Shahida Mohd-Said, Soelaiman Ima-Nirwana, Xin-Feng Leong. Effect of vitamin E on periodontitis: Evidence and proposed mechanisms of action. J Oral Biosci. 2021 Jun;63(2):97-103. doi: 10.1016/j.job.2021.04.001.
Akio Tada, Hiroko Miura. The Relationship between Vitamin C and Periodontal Diseases: A Systematic Review. Int J Environ Res Public Health. 2019 Jul 11;16(14):2472. doi: 10.3390/ijerph16142472.
Ubele van der Velden, Denica Kuzmanova, Iain L Chapple. Micronutritional approaches to periodontal therapy. J Clin Periodontol. 2011 Mar;38 Suppl 11:142-58. doi: 10.1111/j.1600-051X.2010.01663.x.
Sinead Watson, Jayne V Woodside, Lewis Winning, David M Wright, Murali Srinivasan, Gerald McKenna. Associations between self-reported periodontal disease and nutrient intakes and nutrient-based dietary patterns in the UK Biobank. J Clin Periodontol. 2022 May;49(5):428-438. doi: 10.1111/jcpe.13604.
Johan Peter Woelber, Katherina Reichenbächer, Tara Groß, Kirstin Vach, Petra Ratka-Krüger, Valentin Bartha. Dietary and Nutraceutical Interventions as an Adjunct to Non-Surgical Periodontal Therapy-A Systematic Review. Nutrients. 2023 Mar 22;15(6):1538. doi: 10.3390/nu15061538.
Biographies

Denica Kuzmanova is assistant professor and clinical course co-ordinator at the Department of Periodontology, Oral Medicine and Oral Surgery at the Charité – Universitätsmedizin Berlin. She attained her DDS at the University of Hamburg (Germany) and has a doctoral degree in Dentistry (Dr. med. dent.) from the same University. Dr Kuzmanova received speciality training in periodontology and oral implantology as well as her MSc in Periodontology at ACTA, University of Amsterdam (the Netherlands). She has lectured and published in the field of periodontology and is currently a board member of the Berlin Society of Periodontology (BG PARO).

Henrik Dommisch is currently full clinical professor of periodontology and chair of the Department of Periodontology, Oral Medicine and Oral Surgery at the Charité – Universitätsmedizin Berlin. He studied dentistry at the University of Kiel, Germany (DDS degree) and received his training in periodontology and endodontics at the Department of Periodontology, Operative and Preventive Dentistry at the University Hospital Bonn, Germany. In 2004, he received the doctoral degree in dentistry (Dr. med. dent.) from the University of Kiel (Germany) and, in 2008, he finished his habilitation (PhD, Venia legendi) at the University of Bonn (Germany). In 2006 and 2007, Prof. Dommisch worked as a postdoctoral fellow in the Department of Oral Biology at the University of Washington, WA, USA. From 2002 to 2014, he was assistant professor and associate professor in the Department of Periodontology, Operative and Preventive Dentistry at the University Hospital Bonn, Germany. From 2007 to 2019, he was an affiliate professor in the Department of Oral Health Sciences at the University of Washington, WA, USA. In 2004, Prof. Dommisch was appointed as a full professor of periodontology at the Charité – Universitätsmedizin Berlin (Germany). He has lectured and published in the field of periodontology as well as oral biology and received numerous scientific awards. Prof. Dommisch is the current president of the German Society of Periodontology (DG PARO) as well as the Berlin Society of Periodontology (BG PARO).