Therapy, Clinical & Translational Research, Treatment, Article
Which host-modulating approaches are showing most promise?
20 April 2023
Although host-modulating agents can play an adjunctive role in supporting conventional non-surgical therapy for periodontitis, no current approach is recommended in the EFP’s clinical practice guideline on the treatment of periodontitis. Georgios N. Belibasakis and Nagihan Bostanci from the Karolinska Institute in Stockholm explore the latest developments and focus on three promising approaches.
Periodontitis is a disease of microbial aetiology and inflammatory pathogenesis. Conventional non-surgical treatment has the practical aim of removing the biofilm, which is the main trigger of the destructive inflammatory process. Yet the already instigated inflammation is a biological process that takes longer to resolve following the removal of the biofilm and calculus. Furthermore, the resolution time varies significantly between patients and there are different degrees of efficiency.
It is therefore reasonable that scientific and clinical efforts should be made to find active ways to reduce inflammation. This approach — using host-modulating adjuncts in conjunction with subgingival instrumentation of the biofilm and calculus — is considered a part of the second step of periodontal therapy and is addressed in the EFP’s S3 Level Clinical Practice Guideline (CPG) for the treatment of Stage I-III periodontitis.
The evidence was provided in a systematic review commissioned by the EFP (Donos et al., 2020), which gathered the available randomised control trials (RCTs) and, where appropriate, performed systematic meta-analyses. The quality of the evidence was assessed and voted on at the EFP’s Perio Workshop 2019 and the concluding recommendations were reported in the guideline, which was published in the federation’s Journal of Clinical Periodontology (Sanz et al., 2020).
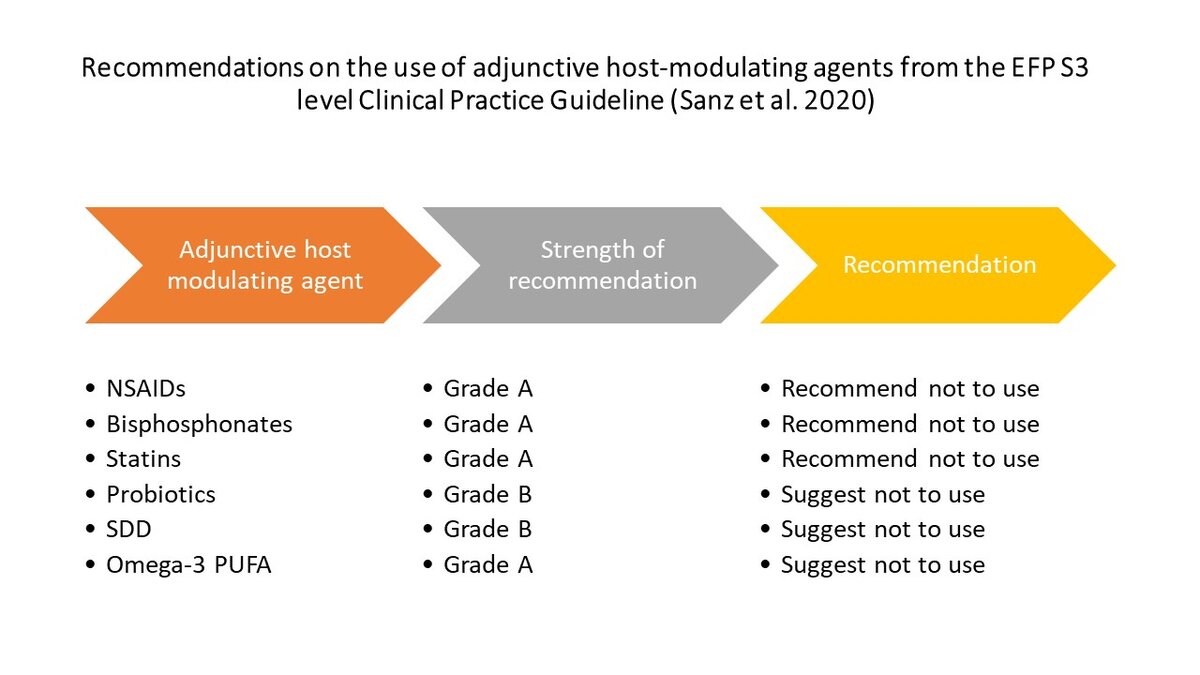
Fewer than a dozen host-modulating approaches were readily addressed in the literature and considered for further assessment. The guideline recommended not to use the following approaches as an adjunct to subgingival instrumentation:
- locally or systemically administered statins
- bisphosphonates
- metformin
- non-steroidal anti-inflammatory drugs (NSAIDs)
- omega−3 polyunsaturated fatty acids (PUFAs).
The guideline also suggested not using probiotics or systemic sub-antimicrobial dose doxycycline (SDD) as an adjunct to subgingival instrumentation. [The CPG format has three grades of recommendation: (1) Strong recommendation (“we recommend”/ “we recommend not to”; (2) Recommendation (“we suggest to” / “we suggest not to”); (3) Open recommendation (“may be considered”).]
There were various reasons why these conclusions were reached: the lack of a sufficient number of adequately powered RCTs to perform a meta-analysis, the absence of multi-centre studies (when all published studies come from a single centre), clinically meaningless benefit compared to the use of placebo, potential side effects that outweigh the potential benefits, or the increased cost of the adjunct that must be borne by the patient.
For the future, we would like to bring forward three of the modalities that currently hold promise as adjuncts to non-surgical periodontal treatment, yet need further follow-up studies and multicentred studies before being re-evaluated for future inclusion in clinical practice guidelines: probiotics, SDD, and resolvins.
Probiotics
Probiotics are defined as “live microorganisms, most commonly bacteria or yeast, which when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014). A few strains and administration protocols have been tested as oral probiotics including Lactobacillus reuteri, Steptococcus salivarius,and Bifididobacterium. It is still unclear how probiotics act on the oral microbiome and how they affect the oral cavity (Hill et al., 2014).
The use probiotics as an adjunctive treatment makes sense from an ecological perspective, as these microorganisms may not only balance out the regional microbial ecology but also prime the local host for a more effective anti-inflammatory response (Ng et al., 2021). There is recent systematic evidence in the literature that probiotics can be an acceptable alternative to conventional antimicrobials in periodontal clinical applications (Henrique Soares et al., 2023).
But the scarcity of adequately powered RCTs with sufficient follow-up, together with the substantial variation in protocols and treatment outcomes between studies, meant that the systematic review performed for Perio Workshop 2019 could not recommend the routine use of probiotics (Donos et al., 2020).
More multicentred and robust RCTs — preferably devoid of commercial interests and using agreed dosage patterns as well as microbial formulations of probiotics — need to be performed to further demonstrate their efficiency and consumer convenience.
It is possible that, given the variations between people’s oral microbiomes, certain formulations may be efficient for some individuals but not for others. Individuals may also respond differently to the same probiotic based on their diet, genetics, systemic health state, and lifestyle. In this context, there would need to be a shift from a “one-size-fits-all” model to a person- and disease-tailored approach (Belibasakis, Bostanci, Marsh, & Zaura, 2019; Bostanci & Belibasakis, 2023).
SDD
It has been documented since the 1980s that doxycycline, apart from its antimicrobial properties, can inhibit collagenolytic activity and proinflammatory cytokines (Bostanci et al., 2011; Golub et al., 1984; Preshaw, 2018). The dosage involved in systemic sub-antimicrobial dose doxycycline is much lower than in its antibiotic formulations (20mg versus 50-100mg).
SDD was introduced as an adjunctive therapeutic modality for the management of periodontitis in the mid-1990s. The fact that adjunctive periodontal treatment with systemically administered SDD has resulted in a significant reduction of deep pockets (≥7mm), renders this adjunctive approach worth considering further (Donos et al., 2020).
The rationale for considering SDD as a host-modulatory rather than an antimicrobial adjunct is its anti-inflammatory and anti-collagenolytic properties (i.e., its capacity to inhibit proinflammatory cytokine production and matrix metalloproteinase activity). Most study protocols used a three-month regimen and did not report any serious adverse effects attributed to the medication, compared to the placebo.
Concerns regarding the use of SDD as an adjunctive therapy have been related to patient compliance and the potential development of antimicrobial resistance over the rather lengthy treatment period — although this has not been indicated in earlier investigations.
Therefore, to move forward with this approach, additional RCTs will be needed to better define the drug administration protocols, the formulations available in the market, and to thoroughly investigate the potential for development of antimicrobial resistance, using cutting-edge technologies, such as resistome analysis (Belibasakis et al., 2020).
Resolvins
Resolvins are endogenous lipid mediators derived from PUFAs, which have the capacity to actively regulate the resolution of inflammation and counteract proinflammatory mediators (Serhan, 2017). Their prospective use as host-modulating adjuncts in the non-surgical treatment of periodontitis is rational, given the inflammatory immunopathogenesis of the disease, and is strongly supported by the fact that the clinical signs of inflammation are only resolved days after the mechanical removal of the microbial biofilm and calculus by scaling and root planing.
There is good evidence that resolvins (or mimetics of resolvins that can tweak the natural inflammatory process) are an efficient treatment modality in experimental animal models (Hasturk et al., 2006), but there are few RCTs available to make the leap to a clinical setting (Donos et al., 2020). Multicentred RCTs are needed to evaluate the long-term outcomes of resolvins as a novel adjunctive treatment modality and to exclude any potential side effects.
At present, these three agents — probiotics, SDD, and resolvins — hold most promise as host-modulating adjunctive treatments for periodontal therapy, while additional options may arise in the future.
However, before any such agent can become readily available for the clinical practitioner it should be integrated into the clinical practice guidelines. But for that to be possible, sufficiently sized and multicentred RCTs need to be available for evaluation, to prove a greater and meaningful clinical outcome in relation to placebo controls, and to validate a safe, convenient, and economic use of a proposed host-modulating agent in any given patient.
Select bibliography
Belibasakis, G. N., Bostanci, N., Marsh, P. D., & Zaura, E. (2019). Applications of the oral microbiome in personalized dentistry. Arch Oral Biol, 104, 7-12. doi:10.1016/j.archoralbio.2019.05.023
Belibasakis, G. N., Lund, B. K., Kruger Weiner, C., Johannsen, B., Baumgartner, D., Manoil, D., . . . Mitsakakis, K. (2020). Healthcare Challenges and Future Solutions in Dental Practice: Assessing Oral Antibiotic Resistances by Contemporary Point-Of-Care Approaches. Antibiotics (Basel), 9(11). doi:10.3390/antibiotics9110810
Bostanci, N., Akgul, B., Tsakanika, V., Allaker, R. P., Hughes, F. J., & McKay, I. J. (2011). Effects of low-dose doxycycline on cytokine secretion in human monocytes stimulated with Aggregatibacter actinomycetemcomitans. Cytokine, 56(3), 656-661. doi:10.1016/j.cyto.2011.08.039
Bostanci, N., & Belibasakis, G. N. (2023). Precision periodontal care: from omics discoveries to chairside diagnostics. Clin Oral Invest. doi:10.1007/s00784-023-04878-7
Donos, N., Calciolari, E., Brusselaers, N., Goldoni, M., Bostanci, N., & Belibasakis, G. N. (2020). The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J Clin Periodontol, 47 Suppl 22, 199-238. doi:10.1111/jcpe.13232
Golub, L. M., Ramamurthy, N., McNamara, T. F., Gomes, B., Wolff, M., Casino, A., . . . et al. (1984). Tetracyclines inhibit tissue collagenase activity. A new mechanism in the treatment of periodontal disease. J Periodontal Res, 19(6), 651-655. doi:10.1111/j.1600-0765.1984.tb01334.x
Hasturk, H., Kantarci, A., Ohira, T., Arita, M., Ebrahimi, N., Chiang, N., . . . Van Dyke, T. E. (2006). RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J, 20(2), 401-403. doi:10.1096/fj.05-4724fje
Henrique Soares, K., Firoozi, P., Maria de Souza, G., Beatriz Lopes Martins, O., Gabriel Moreira Falci, S., & Rocha Dos Santos, C. R. (2023). Efficacy of Probiotics Compared to Chlorhexidine Mouthwash in Improving Periodontal Status: A Systematic Review and Meta-Analysis. Int J Dent, 2023, 4013004. doi:10.1155/2023/4013004
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., . . . Sanders, M. E. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol, 11(8), 506-514. doi:10.1038/nrgastro.2014.66
Ng, E., Tay, J. R. H., Ong, M. M. A., Bostanci, N., Belibasakis, G. N., & Seneviratne, C. J. (2021). Probiotic therapy for periodontal and peri-implant health - silver bullet or sham? Benef Microbes, 12(3), 215-230. doi:10.3920/BM2020.0182
Preshaw, P. M. (2018). Host modulation therapy with anti-inflammatory agents. Periodontol 2000, 76(1), 131-149. doi:10.1111/prd.12148
Sanz, M., Herrera, D., Kebschull, M., Chapple, I., Jepsen, S., Beglundh, T., . . . Methodological, C. (2020). Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol, 47 Suppl 22(Suppl 22), 4-60. doi:10.1111/jcpe.13290
Serhan, C. N. (2017). Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J, 31(4), 1273-1288. doi:10.1096/fj.201601222R
Biographies

Georgios N. Belibasakis
Professor Georgios Belibasakis is head of section in oral health and periodontology, head of the division of oral diseases, and head of research at Karolinska Institutet (Sweden). He attained his DDS at Aristotle University of Thessaloniki (Greece), his MSc in periodontology at Queen Mary University of London (UK), a PhD in oral microbiology at Umeå University (Sweden) and did his post-doctoral studies at King’s College London (UK). He attained his habilitation (docentur) and first professorship in oral microbiology and immunology at the University of Zürich (Switzerland).

Nagihan Bostanci
Nagihan Bostanci is professor in inflammation research (periodontics), director of the master’s degree programme in dentistry and dental hygiene, and head of internationalisation in dentistry at Karolinska Institutet. She has a PhD in periodontology (University of London, UK) and received specialty training in periodontology (Ege University in Turkey and UCL Eastman Dental Institute in the UK). She led the oral translational research group and attained her habilitation in oral biology and periodontology (University of Zürich, Switzerland). She served as president of the IADR/AADR periodontal research group in 2019-2020.